��Ŀ����
��ȤС���ͬѧ��ʵ�������ռ�һͰ����FeSO4��CuSO4�ķ�Һ����������л��ս���ͭ�������������壬��������·�����
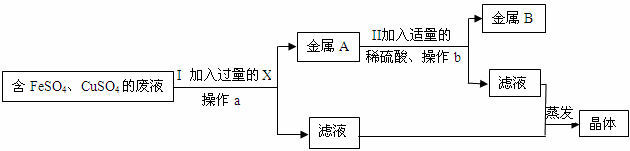
��1������a��b�������� _________ ����Ŀ���dz�ȥ��Һ�� _________ ��������з�����Ӧ�Ļ�ѧ����ʽΪ _________ ��
��2����ͬѧ���X���ý���������ͬѧ��ΪX���ý���п������Ϊ˭���������ȷ�ģ������� _________ ����������Ľ���X��Ŀ���� _________ ��
��3����Һ1����Һ2������Ƿ���ȫ��ͬ�� _________ �������һ�������Ի�þ��崿���Ƿ���Ӱ�죬������ _________ ��
��2����ͬѧ���X���ý���������ͬѧ��ΪX���ý���п������Ϊ˭���������ȷ�ģ������� _________ ����������Ľ���X��Ŀ���� _________ ��
��3����Һ1����Һ2������Ƿ���ȫ��ͬ�� _________ �������һ�������Ի�þ��崿���Ƿ���Ӱ�죬������ _________ ��
��1�����ˣ��������ʣ�Fe+H2SO4=FeSO4+H2����
��2����п�����������ʣ��ò��������������壻ʹ����ͭ�е�ͭȫ���û�������
��3����ͬ�������ᾧ������
��2����п�����������ʣ��ò��������������壻ʹ����ͭ�е�ͭȫ���û�������
��3����ͬ�������ᾧ������

��ϰ��ϵ�д�
�����Ŀ


