��Ŀ����
 ij��ѧ��ȤС�������Һ���ƺʹ��γ����ᴿʵ�飮
ij��ѧ��ȤС�������Һ���ƺʹ��γ����ᴿʵ�飮����������ͼ����ʾ��������һ�����������������Ȼ�����Һ��
��1��ͼ������һ��������������������
��2��ͼ���еIJ���������
��3����ȡ����ˮ�����������ͼ����ʾ�����ʵ��С��ԭ�ƻ����Ƶ��Ȼ�����Һ��������������Ϊ��ˮ���ܶ�Ϊ1g/cm3��
���εij����ᴿʵ��
��ˮ��ɹ�Ρ��õ��Ĵ����к�������ɳ��Ϊ��ȥ�����е���ɳ�������������ͼ����ʾ��ʵ�������
��1��ͼ���б�����������Ʒֱ��ǣ�
a��
��2���������������Ʒֱ���
��3���������е�һ�����Դ�����
��4����������������
���㣺һ������������������Һ������,���˵�ԭ������������Ӧ��,�Ȼ���������ᴿ
ר�⣺��Һ����Һ���ܽ��
��������
��1����������������������һ������Һ�IJ�����������Ҫ��������
��2������������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ����з����жϣ����λ�÷ŷ����������̵�����=���̵�����+������������е�ʽ���м��㣮
��3���������ʵ���������=
��100%����������
��
��1������ʵ���ҳ����������
��2�����ݴ����ᴿ�Ļ�������������ܽ⡢���ˡ�������
��3�����ݹ���ʱ��ע��������
��4����������ʱ���������д�����������ʱ
��1����������������������һ������Һ�IJ�����������Ҫ��������
��2������������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ����з����жϣ����λ�÷ŷ����������̵�����=���̵�����+������������е�ʽ���м��㣮
��3���������ʵ���������=
| �������� |
| ��Һ���� |
��
��1������ʵ���ҳ����������
��2�����ݴ����ᴿ�Ļ�������������ܽ⡢���ˡ�������
��3�����ݹ���ʱ��ע��������
��4����������ʱ���������д�����������ʱ
����⣺
��
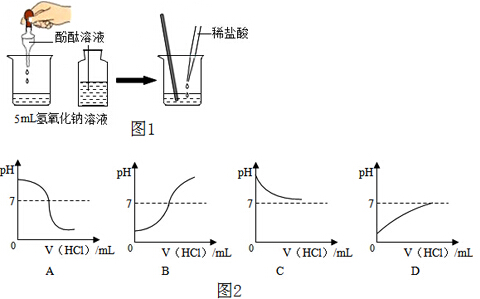
��1������һ�����������������Ȼ�����Һ�����ȼ���������Һ�����Ȼ��ƺ�ˮ���������ٳ���������Ȼ�����ȡˮ���������ܽ⣻����Щ��������Ҫ��������������ƽ��ҩ�ס���Ͳ����ͷ�ιܡ��ձ��Ͳ�������ͼ1��ȱ��һ�ֱ����õ��IJ��������Dz�������
��2��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ����ʾ����������ҩƷλ�÷ŷ��ˣ�
����ͼ2�����������̵�����=���̵�����+�����������֪����������=ҩƷ����+���������������ҩƷ����=��������-������������ҩƷ����=55g-3g=12g��
��3����ʵ��С��ԭ�ƻ���ȡ�Ȼ��Ƶ�����Ϊ15g+3g=18g����ȡ����ˮ�����������ͼ3��ʾ������ȡˮ�����Ϊ82mL=82cm3��������Ϊ1g/cm3��82cm3=82g�������ʵ��С��ԭ�ƻ����Ƶ��Ȼ�����Һ��������������Ϊ
��100%=18%��
��
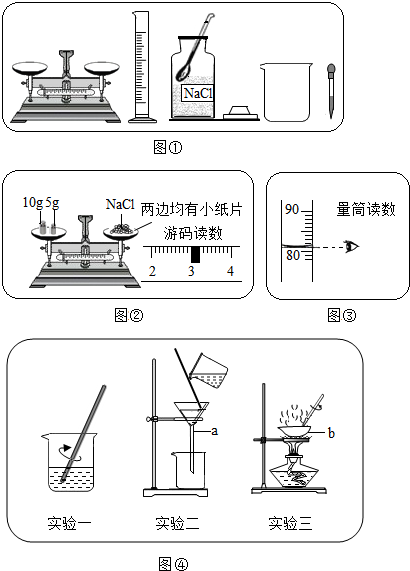
��1������ʵ���ҳ���������֪��a©����b������
��2�������ᴿ�Ļ�������������ܽ⡢���ˡ�������
��3��©���¶�û�н����ձ��ڱڣ�����Һ��������
��4������ʱ���������д�����������ʱֹͣ�����������Ƚ�ˮ�����ɣ�ȡ��bʱ���õ�����������������ǯ��
�ʴ𰸣�
��
��1��ҩ�ף���������
��2��������ҩƷλ�÷ŷ��ˣ�
��3��18%
��
��1��a©����b������
��2���ܽ� ���� ������
��3��©���¶�û�н����ձ��ڱڣ�
��4�����������д�����������ʱ ����ǯ
��
��1������һ�����������������Ȼ�����Һ�����ȼ���������Һ�����Ȼ��ƺ�ˮ���������ٳ���������Ȼ�����ȡˮ���������ܽ⣻����Щ��������Ҫ��������������ƽ��ҩ�ס���Ͳ����ͷ�ιܡ��ձ��Ͳ�������ͼ1��ȱ��һ�ֱ����õ��IJ��������Dz�������
��2��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ����ʾ����������ҩƷλ�÷ŷ��ˣ�
����ͼ2�����������̵�����=���̵�����+�����������֪����������=ҩƷ����+���������������ҩƷ����=��������-������������ҩƷ����=55g-3g=12g��
��3����ʵ��С��ԭ�ƻ���ȡ�Ȼ��Ƶ�����Ϊ15g+3g=18g����ȡ����ˮ�����������ͼ3��ʾ������ȡˮ�����Ϊ82mL=82cm3��������Ϊ1g/cm3��82cm3=82g�������ʵ��С��ԭ�ƻ����Ƶ��Ȼ�����Һ��������������Ϊ
| 18g |
| 18g+82g |
��
��1������ʵ���ҳ���������֪��a©����b������
��2�������ᴿ�Ļ�������������ܽ⡢���ˡ�������
��3��©���¶�û�н����ձ��ڱڣ�����Һ��������
��4������ʱ���������д�����������ʱֹͣ�����������Ƚ�ˮ�����ɣ�ȡ��bʱ���õ�����������������ǯ��
�ʴ𰸣�
��
��1��ҩ�ף���������
��2��������ҩƷλ�÷ŷ��ˣ�
��3��18%
��
��1��a©����b������
��2���ܽ� ���� ������
��3��©���¶�û�н����ձ��ڱڣ�
��4�����������д�����������ʱ ����ǯ
�����������ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢��������������������������йؼ��������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��������ʵ������ȡ�����ķ�Ӧ���ǷֽⷴӦ |
| B������ˮ���ռ�����ʱ�����ܿڸ�������ð�����ռ����� |
| C�������������ȼ��ʱ������ƿ�з�����ˮ�������ն������� |
| D��ʵ�����ù��������������ķ�Ӧ�У���������������� |
������������ʼ�ʱ���ٸ����Ϊ������ڰ˴��漣��ͬѧ��ģ����ٸ�����������£��������ڻ�ѧ�仯���ǣ�������
A�� ��� |
B�� ���� |
C�� ���� |
D�� �ս� |
����ժ¼����ijͬѧ����ѧ֪ʶ���������ɣ�����Ϊ��ȷ���ǣ�������
| A����ʢ��Ũ�����Ũ������Լ�ƿƿ������ƿ�ڶ��а��� |
| B����ʳ����ʪ��pH��ֽ�ϣ��ⶨ��pH |
| C�������ڻ�ѧ��Ӧ��һ���ܼӿ컯ѧ��Ӧ���� |
| D���÷���ˮ������Ӳˮ����ˮ |
һ���¶��£���Ca��OH��2������Һ�м����������ʣ���Һ��pHû�����Ա仯���ǣ�������
| A��CO2 |
| B��CaO |
| C��MgCl2 |
| D��H2SO4 |
��Ҫ��ȥһ����̼�л��е�����������̼�����Ӧ���õķ����ǣ�������
| A������������ȼ |
| B�����������ͨ������ʯ��ˮ |
| C����ѹ����ʹ������̼��Ϊ�ɱ� |
| D�������������һ�������㵹����һ�������� |