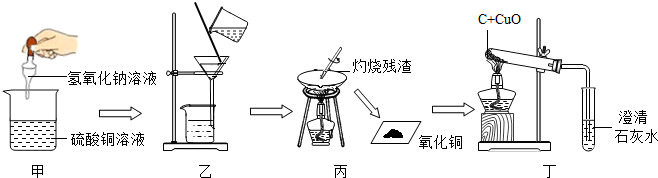
��Ŀ����
�������ƹ�������ܷⲻ�ϣ��ᷢ�����ʣ�Ϊ̽��ѧУʵ������ijƿ�������ƹ�������������ѧ��ȤС�����������ʵ�飺
��1��ȡ������ƿ������Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�������С��ȷ�����������Ѿ����ʣ���ô��С��ͬѧ�������ɫ��Һ������
��2��Ϊ�˼���̽���������Ƶı��ʳ̶ȣ�����ȤС��ͬѧȡ��10g��Ʒ����������ˮ�����Һ��Ȼ������Һ�еμӺ������������Ƶ���Һ��ǡ�õõ�1g������500g��Һ�����������������Һ�����ʵ���������Ϊ���٣�����������ȷ��0.1%��
��1��ȡ������ƿ������Ʒ�����Թ��У������м���һ����ɫ��Һ�����������ݲ�������С��ȷ�����������Ѿ����ʣ���ô��С��ͬѧ�������ɫ��Һ������
��2��Ϊ�˼���̽���������Ƶı��ʳ̶ȣ�����ȤС��ͬѧȡ��10g��Ʒ����������ˮ�����Һ��Ȼ������Һ�еμӺ������������Ƶ���Һ��ǡ�õõ�1g������500g��Һ�����������������Һ�����ʵ���������Ϊ���٣�����������ȷ��0.1%��
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���,��Ļ�ѧ����
ר�⣺�������������뻯ѧ����ʽ���ϵļ���,�����ļ� ���ͨ��
��������1���������������������̼��Ӧ����̼���ƺ�ˮ���������Ʊ��ʱ��̼���ƣ�̼���ƺ�ϡ�����ϡ���ᷴӦ�������������̼���
��2������̼���ƺ��������Ʒ�Ӧ����̼��Ƴ�����ˮ�����ԭ��Һ��̼���Ƶ����������ɵ��������Ƶ��������Ӷ����ԭ��Һ���������Ƶ��������������������Һ�����ʵ�������Ȼ�������������������⼴�ɣ�
��2������̼���ƺ��������Ʒ�Ӧ����̼��Ƴ�����ˮ�����ԭ��Һ��̼���Ƶ����������ɵ��������Ƶ��������Ӷ����ԭ��Һ���������Ƶ��������������������Һ�����ʵ�������Ȼ�������������������⼴�ɣ�
����⣺��1�����������������̼��Ӧ����̼���ƺ�ˮ���������Ʊ��ʱ��̼���ƣ�̼���ƺ�ϡ�����ϡ���ᷴӦ�������������̼��
��2����10g��Ʒ��̼���Ƶ�����Ϊx�������������Ƶ�����Ϊy��
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
106 100 80
x 1g y
=
��
=
x=1.06g��y=0.8g
ԭ�������������Ƶ�����Ϊ10g-1.06g=8.84g��������Һ�����������Ƶ���Һ��������Һ���������Ƶ�����Ϊ8.84g+0.8g=9.64g��
������Һ��������������Ϊ
��100%=1.9%
��������Һ��������������Ϊ1.9%��
�𰸣���1��ϡ���ᣬ��2��1.9%��
��2����10g��Ʒ��̼���Ƶ�����Ϊx�������������Ƶ�����Ϊy��
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
106 100 80
x 1g y
| 106 |
| x |
| 100 |
| 1g |
| 100 |
| 1g |
| 80 |
| y |
x=1.06g��y=0.8g
ԭ�������������Ƶ�����Ϊ10g-1.06g=8.84g��������Һ�����������Ƶ���Һ��������Һ���������Ƶ�����Ϊ8.84g+0.8g=9.64g��
������Һ��������������Ϊ
| 9.64g |
| 500g |
��������Һ��������������Ϊ1.9%��
�𰸣���1��ϡ���ᣬ��2��1.9%��
���������⿼���˸��ݻ�ѧ����ʽ�ļ��㣬Ҫ������������ε����ʲ���ȷ������

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
���б仯�У����������仯���ǣ�������
| A��ţ�̱��� | B����ˮ�ӷ� |
| C������ȼ�� | D��Ŵ����� |
�����й���ϵͳĿǰ�Ѿ��ƹ�ʹ�ý��ܼ��ŵ�˫ȼ�Ϲ���������ͼΪ������ȼ����Ҫ�ɷ���ȫȼ�յĻ�ѧ��Ӧ����ʾ��ͼ��������˵������ȷ���ǣ�������
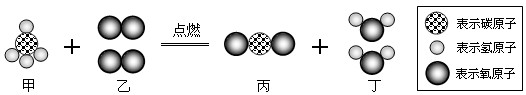

| A���÷�Ӧ�з��ӡ�ԭ�ӵ���������˸ı� |
| B�����ʼĻ�ѧʽ��CH4 |
| C���������е�Ԫ�ػ��ϼ۳�-2�� |
| D��ͼʾ��Ӧ���ڻ��Ϸ�Ӧ |