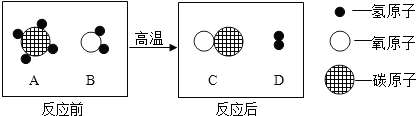
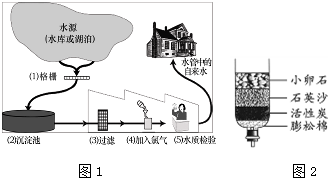
��Ŀ����
�̷�������ˮ����������FeSO4?7H2O��������;�㷺�������������Ρ����������ϡ���ˮ�������������������ȣ�ҽҩ������ƶѪҩ��ũҵ�Ͽ���ũҩ��Ҳ���������ϣ�
��ʵ��һ���̷���FeSO4?7H2O����һ���ۺ����ù������£�
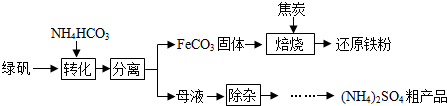
��1���̷���NH4HCO3����Һ�з�Ӧ����CO2���ɣ���Ӧ�Ļ�ѧ����ʽΪ ��
��2�������������С����ա�����Ԫ�ص�ת��;����FeCO3
FeO
Fe
��ʵ������ԭ������CO��д�������ա������и�����Ӧ�Ļ�ѧ����ʽ��
��1��FeCO2
FeO+CO2����
��2�� ��
��3�� ��
��3��ĸҺ�����ӡ������õ���NH4��2SO4�ֲ�Ʒ��������������Ϊ����Ũ���� �����ˡ�ϴ�ӡ����
��ʵ�����Ϊ̽���̷����ȷֽⷴӦ�IJ��ijʵ��С���������ͼ��ʾ��ʵ�飺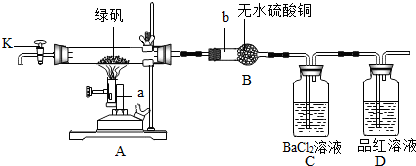
�����ϡ���
��1��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�BaCl2+SO3+H2O=BaSO4��+2HCl����
��2��SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ��
ʵ�鲽�裺
����װ������ ��
�ڽ�һ�������̷���������Ӳ�ʲ������ڣ�����K��ͨ��һ�����ĵ�����
�۹رջ���K����ǿ��ʹ�̷���ȫ�ֽ⣻
�ܴ���K�� ��
�ݼ�¼�۲쵽��ʵ�����������ۣ�
�Իش�
��4��д������ʵ�����������ƣ�a b
��5��������ʵ�鲽�貹���������� ��
��6����ʵ���¼��������з�����
��Ӳ�ʲ���������ɫ��ĩ��ɺ���ɫ��˵���������� ��
��B������ͭ�ɰ�ɫ����ɫ��˵���������� ��
��C�е��������а�ɫ�������ɣ�˵���� ���ɣ�
��D�е�ʵ�������� ��˵����SO2���ɣ�
��7������ʵ����������д���̷����ȷֽ�Ļ�ѧ����ʽ ��
��8�������ⶨ��C�����ɵij���������Ϊ4.66g����μӷ�Ӧ���̷���Ʒ����������д��������̣�
��9����ʵ��С��ͬѧ��Ƶ�ʵ�������һ�����ԵIJ��㣬������Ľ����� ��
��ʵ��һ���̷���FeSO4?7H2O����һ���ۺ����ù������£�

��1���̷���NH4HCO3����Һ�з�Ӧ����CO2���ɣ���Ӧ�Ļ�ѧ����ʽΪ
��2�������������С����ա�����Ԫ�ص�ת��;����FeCO3
| �ֽ� |
| ��ԭ |
��ʵ������ԭ������CO��д�������ա������и�����Ӧ�Ļ�ѧ����ʽ��
��1��FeCO2
| ||
��2��
��3��
��3��ĸҺ�����ӡ������õ���NH4��2SO4�ֲ�Ʒ��������������Ϊ����Ũ����
��ʵ�����Ϊ̽���̷����ȷֽⷴӦ�IJ��ijʵ��С���������ͼ��ʾ��ʵ�飺

�����ϡ���
��1��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�BaCl2+SO3+H2O=BaSO4��+2HCl����
��2��SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ��
ʵ�鲽�裺
����װ������
�ڽ�һ�������̷���������Ӳ�ʲ������ڣ�����K��ͨ��һ�����ĵ�����
�۹رջ���K����ǿ��ʹ�̷���ȫ�ֽ⣻
�ܴ���K��
�ݼ�¼�۲쵽��ʵ�����������ۣ�
�Իش�
��4��д������ʵ�����������ƣ�a
��5��������ʵ�鲽�貹����������
��6����ʵ���¼��������з�����
��Ӳ�ʲ���������ɫ��ĩ��ɺ���ɫ��˵����������
��B������ͭ�ɰ�ɫ����ɫ��˵����������
��C�е��������а�ɫ�������ɣ�˵����
��D�е�ʵ��������
��7������ʵ����������д���̷����ȷֽ�Ļ�ѧ����ʽ
��8�������ⶨ��C�����ɵij���������Ϊ4.66g����μӷ�Ӧ���̷���Ʒ����������д��������̣�
��9����ʵ��С��ͬѧ��Ƶ�ʵ�������һ�����ԵIJ��㣬������Ľ�����
���㣺���ʵ��ת�����Ʊ�,��������ļ�������ӷ���,�εĻ�ѧ����,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺���ʵ��Ʊ�
��������ʵ��һ����1�����ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ������Ȼ�����������غ㶨����д��ѧ����ʽ��
��2�����ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ������Ȼ�����������غ㶨����д��ѧ����ʽ��
��3����������茶�����ܽ�����߽��з�����
�����ϡ���4��������������״�����ý��з�����
��5�����ݸ�װ�������������壬Ҳ��Ҫ����������з�����
��6�����������غ㶨�ɡ��������Ǻ�ɫ��ĩ��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ���з�����
��7�����ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ������Ȼ�����������غ㶨����д��ѧ����ʽ��
��8���������е�ת����ϵ�ҳ��̷���Ʒ��C�еij����Ĺ�ϵʽ��Ȼ�����C�еij�������������̷���Ʒ��������
��9�����ݶ�������������������Ⱦ�������з�����
��2�����ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ������Ȼ�����������غ㶨����д��ѧ����ʽ��
��3����������茶�����ܽ�����߽��з�����
�����ϡ���4��������������״�����ý��з�����
��5�����ݸ�װ�������������壬Ҳ��Ҫ����������з�����
��6�����������غ㶨�ɡ��������Ǻ�ɫ��ĩ��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ���з�����
��7�����ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ������Ȼ�����������غ㶨����д��ѧ����ʽ��
��8���������е�ת����ϵ�ҳ��̷���Ʒ��C�еij����Ĺ�ϵʽ��Ȼ�����C�еij�������������̷���Ʒ��������
��9�����ݶ�������������������Ⱦ�������з�����
����⣺��ʵ��һ����1����ˮ����������̼����立�Ӧ����̼����������李�ˮ�Ͷ�����̼����ѧ����ʽΪ��FeSO4?7H2O+2NH4HCO3=FeCO3+��NH4��2SO4+CO2��+8H2O��
��2��������̼��ľ̿�ڸ��µ�����������һ����̼����ѧ����ʽΪ��CO2+C
2CO��
һ����̼�����������ڼ��ȵ��������������Ͷ�����̼����ѧ����ʽΪ��FeO+CO
Fe+CO2��
��3��ͨ����������淋��ܽ�����߿�֪��������ܽ�����¶�Ӱ���ܽ�ȱ仯�ϴ�����Ҫ�õ�����茶�����Ҫ������ȴ�ȱ�����Һ�ķ�����Ȼ���ٽ��й��ˡ�ϴ�ӡ����
�����ϡ���4��ͨ���۲�װ��ͼ�е����������Կ���aװ�����������ȵģ����ƾƾ��ƣ�����a�Ǿƾ���ƣ�bװ������������ģ�b�����θ���ܣ�
��5��ͨ��������ʵ��װ�ã���Ӧ�����������壬��Ҫ�����Ӻ�װ�ú����װ�õ������ԣ�
�÷�Ӧ�������˶��������������������ڷ�Ӧ����Ҫ����Щ�����ų����м��飬���Է�Ӧ����Ҫ��������һ����������
��6����Ӳ�ʲ���������ɫ��ĩ��ɺ���ɫ�����������غ㶨�ɿ�֪������ɫ��ĩ��������������˵���������У�Fe2O3��
����ˮ����ͭ����ˮ�ԣ���ˮ������ɫ��������������ˮ�Ĵ��ڣ�B������ͭ�ɰ�ɫ����ɫ��˵���������У�H2O��
��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�C�е��������а�ɫ�������ɣ�˵����SO3���ɣ�
��SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ�����֤�����ж����������ɣ�����D�е�ʵ�������ǣ�Ʒ����Һ��ɫ��
��7����ˮ���������ڸ��µ������������������������������������ˮ����ѧ����ʽΪ��2FeSO4?7H2O
Fe2O3+SO3��+SO2��+14H2O��
��8���⣺��μӷ�Ӧ���̷���Ʒ����Ϊx��
2FeSO4?7H2O��SO3��BaSO4
556 233
x 4.66g
=
x=11.12g
�𣺲μӷ�Ӧ���̷���Ʒ����Ϊ11.12g��
��9����������������������Ⱦ���������ԸĽ������ǣ���Dװ�ú��β������װ�ã���ֹ��Ⱦ������
�ʴ�Ϊ����ʵ��һ����1��FeSO4?7H2O+2NH4HCO3=FeCO3+��NH4��2SO4+CO2��+8H2O��
��2��CO2+C
2CO��FeO+CO
Fe+CO2��
��3����ȴ�ȱ�����Һ��
�����ϡ���4���ƾ���ƣ����θ���ܣ�
��5�����װ�������ԣ���������һ����������
��6����Fe2O3����H2O����SO3����Ʒ����Һ��ɫ��
��7��2FeSO4?7H2O
Fe2O3+SO3��+SO2��+14H2O��
��8���⣺��μӷ�Ӧ���̷���Ʒ����Ϊx��
2FeSO4?7H2O��SO3��BaSO4
556 233
x 4.66g
=
x=11.12g
�𣺲μӷ�Ӧ���̷���Ʒ����Ϊ11.12g��
��9����Dװ�ú��β������װ�ã���ֹ��Ⱦ������
��2��������̼��ľ̿�ڸ��µ�����������һ����̼����ѧ����ʽΪ��CO2+C
| ||
һ����̼�����������ڼ��ȵ��������������Ͷ�����̼����ѧ����ʽΪ��FeO+CO
| ||
��3��ͨ����������淋��ܽ�����߿�֪��������ܽ�����¶�Ӱ���ܽ�ȱ仯�ϴ�����Ҫ�õ�����茶�����Ҫ������ȴ�ȱ�����Һ�ķ�����Ȼ���ٽ��й��ˡ�ϴ�ӡ����
�����ϡ���4��ͨ���۲�װ��ͼ�е����������Կ���aװ�����������ȵģ����ƾƾ��ƣ�����a�Ǿƾ���ƣ�bװ������������ģ�b�����θ���ܣ�
��5��ͨ��������ʵ��װ�ã���Ӧ�����������壬��Ҫ�����Ӻ�װ�ú����װ�õ������ԣ�
�÷�Ӧ�������˶��������������������ڷ�Ӧ����Ҫ����Щ�����ų����м��飬���Է�Ӧ����Ҫ��������һ����������
��6����Ӳ�ʲ���������ɫ��ĩ��ɺ���ɫ�����������غ㶨�ɿ�֪������ɫ��ĩ��������������˵���������У�Fe2O3��
����ˮ����ͭ����ˮ�ԣ���ˮ������ɫ��������������ˮ�Ĵ��ڣ�B������ͭ�ɰ�ɫ����ɫ��˵���������У�H2O��
��SO2ͨ��BaCl2��Һ������������SO3ͨ��BaCl2��Һ�У��а�ɫ�������ɣ�C�е��������а�ɫ�������ɣ�˵����SO3���ɣ�
��SO2��ʹƷ����Һ��ɫ��SO3������ʹƷ����Һ��ɫ�����֤�����ж����������ɣ�����D�е�ʵ�������ǣ�Ʒ����Һ��ɫ��
��7����ˮ���������ڸ��µ������������������������������������ˮ����ѧ����ʽΪ��2FeSO4?7H2O
| ||
��8���⣺��μӷ�Ӧ���̷���Ʒ����Ϊx��
2FeSO4?7H2O��SO3��BaSO4
556 233
x 4.66g
| 556 |
| x |
| 233 |
| 4.66g |
x=11.12g
�𣺲μӷ�Ӧ���̷���Ʒ����Ϊ11.12g��
��9����������������������Ⱦ���������ԸĽ������ǣ���Dװ�ú��β������װ�ã���ֹ��Ⱦ������
�ʴ�Ϊ����ʵ��һ����1��FeSO4?7H2O+2NH4HCO3=FeCO3+��NH4��2SO4+CO2��+8H2O��
��2��CO2+C
| ||
| ||
��3����ȴ�ȱ�����Һ��
�����ϡ���4���ƾ���ƣ����θ���ܣ�
��5�����װ�������ԣ���������һ����������
��6����Fe2O3����H2O����SO3����Ʒ����Һ��ɫ��
��7��2FeSO4?7H2O
| ||
��8���⣺��μӷ�Ӧ���̷���Ʒ����Ϊx��
2FeSO4?7H2O��SO3��BaSO4
556 233
x 4.66g
| 556 |
| x |
| 233 |
| 4.66g |
x=11.12g
�𣺲μӷ�Ӧ���̷���Ʒ����Ϊ11.12g��
��9����Dװ�ú��β������װ�ã���ֹ��Ⱦ������
�����������ѶȱȽϴ��Ķ����࣬����������ʱ��������Ҫ�������ҳ����õ����ʣ�Ȼ�������е������ѧ����֪ʶ���н��

��ϰ��ϵ�д�
�����Ŀ
 ��ͼ�ǿ�����������������ⶨ��ʵ��װ�ã�
��ͼ�ǿ�����������������ⶨ��ʵ��װ�ã�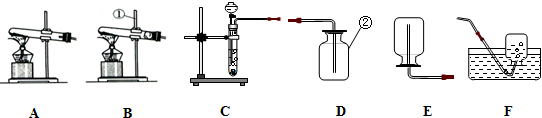
 �ԡ�����--δ����Ϊ�����2012��ʮ�Ľ���������ʳ�չ��3��21����31���ڹ������¹��ʻ�չ�������ľ��У���̼��������ɫ���á��ɳ�����չ�������Ϊ��չ�������ɣ���ش��������⣺
�ԡ�����--δ����Ϊ�����2012��ʮ�Ľ���������ʳ�չ��3��21����31���ڹ������¹��ʻ�չ�������ľ��У���̼��������ɫ���á��ɳ�����չ�������Ϊ��չ�������ɣ���ش��������⣺