��Ŀ����
19�� A��B��C��D�dz��л�ѧ�г��������ʣ�����A��θ�����Ҫ�ɷ֣�B��ũҵ�ϳ����ڸ�������������D������ΪҺ�壮A��B��C��D�����������ͬ���������Ӧ��ת����ϵ��ͼ��ʾ����������ʾ��һ�����ʿ���ת��Ϊ��һ�����ʣ���-����ʾ���ڵ����ʼ�������Ӧ�����в��ַ�Ӧ��������P��Ӧ��������ȥ������ش�
A��B��C��D�dz��л�ѧ�г��������ʣ�����A��θ�����Ҫ�ɷ֣�B��ũҵ�ϳ����ڸ�������������D������ΪҺ�壮A��B��C��D�����������ͬ���������Ӧ��ת����ϵ��ͼ��ʾ����������ʾ��һ�����ʿ���ת��Ϊ��һ�����ʣ���-����ʾ���ڵ����ʼ�������Ӧ�����в��ַ�Ӧ��������P��Ӧ��������ȥ������ش���1��D������ˮ��
��2��д��B��C��Ӧ�Ļ�ѧ����ʽNa2CO3+Ca��OH��2�TCaCO3��+2NaOH��
��3��д��A��C��Ӧ�Ļ�ѧ����ʽHCl+NaOH�TNaCl+H2O��
���� ����Aθ���е���Ҫ�ɷ������ᣬ�����ܺ��������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������B��ũҵ�ϳ����ڸ���������������BΪ�������ƣ��������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ�����ᷴӦ����ˮ���Σ�����D������ΪҺ�壬�����Ϊˮ������ͼ����CΪ�������ƣ�
��� �⣺����A��θ�����Ҫ�ɷ֣���A���������B��ũҵ�ϳ����ڸ���������������BΪ�������ƣ������ܺ��������Ʒ�Ӧ�����Ȼ��ƺ�ˮ������������ũҵ�ϳ����ڸ��������������������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ�����ᷴӦ����ˮ���Σ����ABCD�ֱ������ᡢ�������ơ��������ƺ�ˮ��������飮
��1�����ݷ�����D������ˮ�����ˮ��
��2�����ݷ�������BΪ�������ƣ�C���������ƣ���B��C��Ӧ�����������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ƣ���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��
��3�����ݷ�����A�����ᣬCΪ�������ƣ���������������Ʒ�Ӧ�Ļ�ѧ����ʽΪ��HCl+NaOH�TNaCl+H2O��
�ʴ�Ϊ��
ˮ��Na2CO3+Ca��OH��2�TCaCO3��+2NaOH��HCl+NaOH�TNaCl+H2O��
���� ������Ҫ�������ʵ����ʣ����ʱҪ���ݸ������ʵ����ʣ���ϸ������������з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
������ƿ��Ӧʢ̼��������Һ����ƿ��ӦʢŨ���ᣮ
��2��������������Һ��ͨ������������̼��ʹ��Һ�� 50%���������Ʒ�Ӧ����Ӧ����Һ�� CO${\;}_{3}^{2-}$�� OH�ĸ�����Ϊ1��2��
��3���ֽ�ʯ��ʯ��Ʒ 15g �� 105.4g ϡ��������ձ��г�ַ�Ӧ�����ʲ�����ˮҲ����ϡ ���ᷴӦ������Ӧʱ���뷴Ӧǰ���ձ������ʵ������仯�����ʾ��
| ��Ӧʱ��/s | 20 | 40 | 60 | 80 | 100 | 120 |
| ����/g | 119.52 | 118.64 | 117.76 | 116.88 | 116 | 116 |
�ڷ�Ӧ��������Һ�����ʵ�����������
 �ס��ҡ�����������������һ�������»�Ϸ�Ӧ��һ��ʱ���ø����ʵ����������仯��ͼ��ʾ�����й�˵����ȷ���ǣ�������
�ס��ҡ�����������������һ�������»�Ϸ�Ӧ��һ��ʱ���ø����ʵ����������仯��ͼ��ʾ�����й�˵����ȷ���ǣ�������| A�� | �÷�Ӧ���ڷֽⷴӦ | |
| B�� | ��һ���Ǹ÷�Ӧ�Ĵ��� | |
| C�� | ���ǵ��� | |
| D�� | �÷�Ӧ���ɼͱ���������Ϊ19��41 |
| A�� | 1.6g | B�� | 2.56g | C�� | 3.2g | D�� | 4.8g |
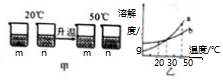
| A�� | ͼ����a��ʾm���ܽ������ | |
| B�� | ͼ��20����Һ�����ʵ���������m����n | |
| C�� | ͼ��50��ʱ��m��n��Һһ���Dz�������Һ | |
| D�� | 30��ʱ��a��b��Һ����������������� |

 A��E�dz��г��������ʣ����ᡢ�������ơ��������ơ�̼���ơ�������̼�е�ijһ�֣�A�����ڽ���������⣬B��������������������������䷢����Ӧ��ת���Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ�������ʼ��ת����ϵ����
A��E�dz��г��������ʣ����ᡢ�������ơ��������ơ�̼���ơ�������̼�е�ijһ�֣�A�����ڽ���������⣬B��������������������������䷢����Ӧ��ת���Ĺ�ϵ��ͼ��ʾ����-����ʾ�������ʼ��ܷ�����Ӧ����������ʾ�������ʼ��ת����ϵ����