��Ŀ����
1�� ijͬѧ�ⶨһƿ��ǩ��ȱ������þ��Һ����������������ȡ60g����Һ����������μ���������������Ϊ10%������������Һ�����ɳ�������������������������Һ��������ϵ��ͼ��ʾ����Ӧ����ʽΪ��
ijͬѧ�ⶨһƿ��ǩ��ȱ������þ��Һ����������������ȡ60g����Һ����������μ���������������Ϊ10%������������Һ�����ɳ�������������������������Һ��������ϵ��ͼ��ʾ����Ӧ����ʽΪ��MgSO4+2NaOH=Na2SO4+Mg��OH��2��
��1������100g������������Ϊ10%��NaOH��Һ����NaOH������Ϊ10g��
��2������MgSO4��Һ�����ʵ�������������ʽ���㣩��
���� ��1��������Һ�����������������������ʵ�������
��2���������ɵ�������þ�������Ͷ�Ӧ�Ļ�ѧ����ʽ��������þ����������������������������
��� �⣺
��1������100g������������Ϊ10%��NaOH��Һ����NaOH������Ϊ100g��10%=10g��
��2����ͼ���Կ������ɵ�������þ������Ϊ2.9g
������þ������Ϊx
MgSO4+2NaOH=Na2SO4+Mg��OH��2��
120 58
x 2.9g
$\frac{120}{58}$=$\frac{x}{2.9g}$
x=6g
MgSO4��Һ�����ʵ���������Ϊ$\frac{6g}{60g}$��100%=10%
��
��1������100g������������Ϊ10%��NaOH��Һ����NaOH������Ϊ 10g��
��2��MgSO4��Һ�����ʵ���������Ϊ10%��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������

��ϰ��ϵ�д�
�����Ŀ
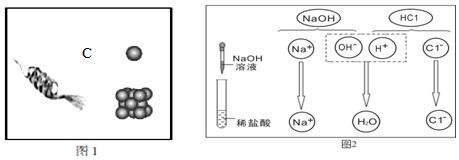
16�����и������ʣ�������������л����˳�����е��ǣ�������
| A�� | ������ˮ������ | B�� | ���ᣬ����þ��ά����C | ||
| C�� | ����֣�Һ�������� | D�� | ʯ�ͣ���������̼���� |
6�����л�ѧ����ʽ����ȷ��ʾ�������ݵ��ǣ�������
| A�� | ֤��ͭ�������ã�Cu+2AgCl�TCuCl2+2Ag | |
| B�� | ������γ�ԭ����CO2+H2O�TH2CO3 | |
| C�� | ��������⣨Fe2O3•nH2O����Fe2O3•nH2O+6HCl�T2FeCl3+��n+3��H2O | |
| D�� | ��ȥH2�л��е�HCl���壺Na2CO3+2HCl�T2NaCl+H2O+CO2�� |