��Ŀ����
2������һ��ij��ѧС��Ϊ̽��������̼����ȡ�����ʣ����������ʵ��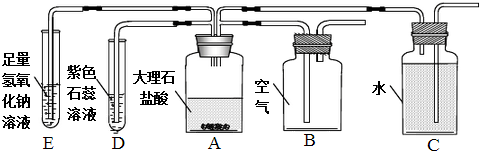
��1��AΪʵ������ȡ������̼��ʵ�飬�÷�Ӧ�Ļ�ѧ����ʽΪCaCO3+2HCl=CaCl2+CO2��+H2O
��2�����û�ѧ����ʽ����D����ɫʯ�����ԭ��
��Ҫ֤������ʯ�к�̼������ӣ�ֻ�轫D���Լ���Ϊ�����ʯ��ˮ�����Լ����ƣ�
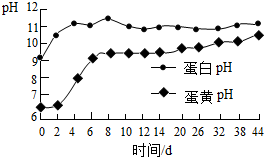
��3�������ַ����ռ�����ʱ�����B��C���������������ʱ��ı仯��ϵ��ͼ����ʾ������Ϊ�ܣ���ܡ����ܡ�������ˮ���ռ�������̼�����й���C�Ĵ�ʩ���ܼ��ٶ�����̼��ˮ���ܽ������Ǣ٢ۣ�����ţ�
����ˮ������һ��ֲ����
���ռ�����ʱ������C
������ˮ�滻��ˮ
��4����֪��������1���ˮ����ܽ�1���������̼���õ�pHֵԼΪ5.6�ı�����Һ��ʵ������������D����ҺpHֵС��5.6�����ҡ�������ͼ����E�б仯����ϵ� ���Ҷ�������ţ�
��5��Ϊ�б�E��NaOH�Ƿ���ʣ�࣬Ӧ��E�м�����Լ��Тܣ�����ţ���
��ϡ����
���Ȼ�����Һ
������������Һ
������ͭ��Һ��
���� ��1����ʵ������ȡ������̼��ԭ�����
��2������̼��ƺ����ᷴӦ����ʹʯ��ˮ����ǵĶ�����̼������
��3������ͼ�ס�������ܽ�Ƚ����жϣ�
��4������E�ж�����̼���������Ʒ�Ӧ����̼���ƺ�ˮ�����ͼ�����з�����
��5��������������������ͭ��Ӧ�������з�����
��� �⣺��1��������̼��ʵ�����Ʒ�����ϡ���������ʯ����ʯ��ʯ������Ҫ�ɷֶ���̼��ƣ���Ӧ����ȡ���䷴Ӧԭ����CaCO3+2HCl=CaCl2+CO2��+H2O��
��2����Ҫ֤������ʯ�к�̼������ӣ�ֻ�轫D���Լ���Ϊ�����ʯ��ˮ����ΪA�в����Ķ�����̼��ʹ�����ʯ��ˮ����ǣ�
��3��ͨ��ͼ��֪������ˮ���ռ�������̼����ˮ������һ��ֲ���Ϳɷ�ֹ������̼����ˮ������������ܽ�����¶ȵ����߶���С������ˮ�滻��ˮ�ɼ��ٶ�����̼��ˮ���ܽ�����
��4����֪��������1���ˮ����ܽ�1���������̼���õ�pHֵԼΪ5.6�ı�����Һ��ʵ������������D����ҺpHֵС��5.6�����ҡ�������ͼ����E�б仯����ϵ����Ҷ���
��5��Ϊ�б�E��NaOH�Ƿ���ʣ�࣬Ӧ��E�м�����Լ�������ͭ��Һ��
�ʴ�Ϊ��
��1��CaCO3+2HCl=CaCl2+CO2��+H2O��
��2�������ʯ��ˮ����3���ܡ��٢ۣ���4���Ҷ�����5���ܣ�
���� ���⿼���˳����������ȡ��������ӡ��ռ�����Ӧԭ������д�ȣ��ۺ��Խ�ǿ�����������������ʲⶨ���ϵ����Ԫ��ʱ��Ҫע�����ָ������������ú�Ŀ�ģ����������غ㶨�ɽ����������ķ�����

| A�� |  ú��ȼ�� | B�� |  ������� | C�� |  ������ʴ | D�� |  �������� |
 Ԫ�����ڱ�����Ԫ�ص�ijЩ��Ϣ��ͼ��ʾ�������й�����˵������ȷ���ǣ�������
Ԫ�����ڱ�����Ԫ�ص�ijЩ��Ϣ��ͼ��ʾ�������й�����˵������ȷ���ǣ�������| A�� | �ǽ���Ԫ�� | B�� | ԭ�Ӻ�����34������ | ||
| C�� | ���ԭ������Ϊ78.96 | D�� | ��������������Ԫ�� |
���������ϡ���
��NaN3��ײ����Ѹ�ٷֽ�����Na��N2��д���÷�Ӧ�Ļ�ѧ����ʽ2NaN3$\frac{\underline{\;ײ��\;}}{\;}$2Na+3N2����
��NaN3�����ᡢH2SO4��Һ���������ɣ�
�ۼ�ʯ����CaO�� NaOH�Ļ���
��NaN3��ҵ���Ʊ������ǣ�����������Һ̬����Ӧ�Ƶ�NaNH2���ٽ�NaNH2��N2O��Ӧ������NaN3��NaOH������X���÷�Ӧ�Ļ�ѧ����ʽΪ2NaNH2+N2O=NaN3+NaOH+X����X�Ļ�ѧʽΪNH3��
������̽��������һ���ⶨij��ҵ��NaN3��Ʒ��Na2CO3����������
��1����ҵ��NaN3�г�����������Na2CO3����ԭ���ǣ��û�ѧ����ʽ��ʾ��2NaOH+CO2�TNa2CO3+H2O��
��2��ijͬѧ�����ͼ1װ�ò��̼���Ƶ�������������ʵ�����ݼ�¼�����
| ������Ŀ | ����ʱ�� | ������g�� |
| ���� | 100.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 339.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 339.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 339.00 |
������Ũ�����ϴ��װ�ã���ʹ�ⶨ�Ľ��ƫ���ƫ����ƫС�����䡱����
�����ñ������ݼ����̼���Ƶ���������Ϊ5.3%��
������̽�������������ⶨij��ҵ����Ʒ��NaN3����������
С̸ͬѧ��ȷ����0.140g NaN3��Ʒ�������ͼ2װ�òⶨ������������
��֪2NaN3��3N2��NaN3�е�Nȫ��ת��ΪN2�����������ԣ�����Ӧ�зų��������ȣ�
��1������װ�������Եķ����ǣ����Ӻ�װ�ã���ˮ��עˮ���������߳����ȶ���Һ��˵�����������ã�
��2��ʹ����ˮ������Ŀ���ǣ�ʹˮ����Һ�������ȥˮ����������
��3����б��ƿʹС�Թ��е�NaN3��Ʒ��M��Һ�Ӵ���ַ�Ӧ�� �����²�������ܶ���Ϊ67.2mL��N2���ܶ�Ϊ1.25g/L������ʵ����ѡ�õ������ܹ����ʵ���A������ĸ��ţ���
A.100mL B.1L C.2L
��4�����㹤ҵ����Ʒ��NaN3������������д��������̣��������0.1%����

| A�� | ��ˮ�ӷ� | B�� | �������� | C�� | ��ѩ�ڻ� | D�� | ұ������ |