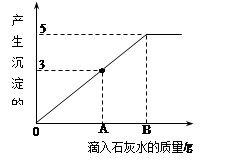��Ŀ����
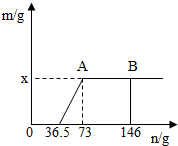
һ�ձ���ʢ��Na2CO3��NaOH�Ļ��������м�����ˮ�ܽ��Ƴ���Һ�����μ�������������Ϊ10%��ϡ���ᣬ�ų������������m�������μ�ϡ�����������n���Ĺ�ϵ��ͼ��ʾ������������ͼʾ����������⣺��1�����μӵ�73gϡ����ʱ���ų����������x=______ g��
��2������д���ձ����Ⱥ�����Ӧ�Ļ�ѧ����ʽ��______��______��
��3�����μ�ϡ������B��ʱ���ձ�����Һ�е������ǣ�д��ѧʽ��______���������ֱ��Ƕ��٣�

���𰸡���������1������ͼʾ����Ϣ��֪������뵽36.5gʱ��ʼ����������̼���������ɶ�����̼���ʱ���õ����������36.5g�����÷���ʽ���������̼��������
��2����Ӧ��ʼû�����ɶ�����̼����������������ķ�Ӧ��Ȼ����������̼���Ƶķ�Ӧ��
��3���μӵ�B�������������Ͽ�ʼʱ������Ӧ�IJ�����ж����ʵĻ�ѧʽ���������ֱ�ɲ������ݽ��з������Ȼ��Ƶ�������ͨ��Ԫ�ص��غ㣬��73g�������ȵĺ������Ȼ������ȵĺ�����ȣ�HCl�����ǴﵽB�������������ﵽA�����õ��������������HCl��������
����⣺��1�������ɵĶ�����̼��������x
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 44
��73-36.5��×10% x
 =
=
x=2.2g
�ʴ�Ϊ��2.2
��2����Ϊ��ʼ��Ӧʱ��������̼���ɣ���������������������ķ�Ӧ�����������˶�����̼����̼����������ķ�Ӧ��
�ʴ�Ϊ��NaOH+HCl�TNaCl+H2O Na2CO3+2HCl�T2NaCl+CO2��+H2O
��3����ͼ���֪�μӵ�B�������������Ͽ�ʼʱ������Ӧ�IJ�����ж��������Ȼ�����HCl���������ֱ�ɲ������ݽ��з������Ȼ��Ƶ�������ͨ����Ӧ��������Ԫ�ص��غ���ɣ���73g�������ȵĺ������Ȼ������ȵĺ�����ȣ�HCl�����ǴﵽB�������������ﵽA�����õ��������������HCl��������
��NaCl������Ϊy����������������Ӧ��ͼʾ���ݿɵã�
HCl----------��NaCl
36.5 58.5
73g×10% y
 =
=
y�T11.7g
m��HCl���T��146g-73g��×10%=7.3g
�ʴ�Ϊ��NaCl HCl �������ֱ��ǣ�11.7g��7.3g��
������������һ���ۺϼ����⣬����Ĺؼ��Ƕ���ص�ͼ����Ϣ�ķ���������������ѵ��ѧ�����õ��ۺ�˼ά�����нϺõİ�����
��2����Ӧ��ʼû�����ɶ�����̼����������������ķ�Ӧ��Ȼ����������̼���Ƶķ�Ӧ��
��3���μӵ�B�������������Ͽ�ʼʱ������Ӧ�IJ�����ж����ʵĻ�ѧʽ���������ֱ�ɲ������ݽ��з������Ȼ��Ƶ�������ͨ��Ԫ�ص��غ㣬��73g�������ȵĺ������Ȼ������ȵĺ�����ȣ�HCl�����ǴﵽB�������������ﵽA�����õ��������������HCl��������
����⣺��1�������ɵĶ�����̼��������x
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 44
��73-36.5��×10% x
 =
=
x=2.2g
�ʴ�Ϊ��2.2
��2����Ϊ��ʼ��Ӧʱ��������̼���ɣ���������������������ķ�Ӧ�����������˶�����̼����̼����������ķ�Ӧ��
�ʴ�Ϊ��NaOH+HCl�TNaCl+H2O Na2CO3+2HCl�T2NaCl+CO2��+H2O
��3����ͼ���֪�μӵ�B�������������Ͽ�ʼʱ������Ӧ�IJ�����ж��������Ȼ�����HCl���������ֱ�ɲ������ݽ��з������Ȼ��Ƶ�������ͨ����Ӧ��������Ԫ�ص��غ���ɣ���73g�������ȵĺ������Ȼ������ȵĺ�����ȣ�HCl�����ǴﵽB�������������ﵽA�����õ��������������HCl��������
��NaCl������Ϊy����������������Ӧ��ͼʾ���ݿɵã�
HCl----------��NaCl
36.5 58.5
73g×10% y
 =
=
y�T11.7g
m��HCl���T��146g-73g��×10%=7.3g
�ʴ�Ϊ��NaCl HCl �������ֱ��ǣ�11.7g��7.3g��
������������һ���ۺϼ����⣬����Ĺؼ��Ƕ���ص�ͼ����Ϣ�ķ���������������ѵ��ѧ�����õ��ۺ�˼ά�����нϺõİ�����

��ϰ��ϵ�д�
�����Ŀ
 һ�ձ���ʢ��Na2CO3��NaOH�Ļ��������м�����ˮ�ܽ��Ƴ���Һ�����μ�������������Ϊ10%��ϡ���ᣬ�ų������������m�������μ�ϡ�����������n���Ĺ�ϵ��ͼ��ʾ������������ͼʾ����������⣺
һ�ձ���ʢ��Na2CO3��NaOH�Ļ��������м�����ˮ�ܽ��Ƴ���Һ�����μ�������������Ϊ10%��ϡ���ᣬ�ų������������m�������μ�ϡ�����������n���Ĺ�ϵ��ͼ��ʾ������������ͼʾ����������⣺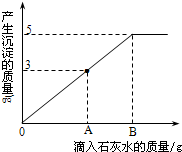 ��2011?���϶�ģ����һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��2011?���϶�ģ����һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺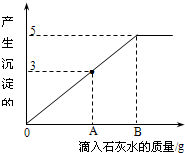 ��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺
��һ�ձ���ʢ��Na2CO3��NaOH�Ļ����10g�������м���100gˮ������ȫ���ܽ⣮��������Һ�еμӳ���ʯ��ˮ���������������������ʯ��ˮ��������ϵ������ͼ��ʾ�����������ش��������⣺