��Ŀ����
�ճ�ʹ�õĽ������ϣ���������ںϽ𣮻�ͭ����п����Ҫ����Ԫ�ص�ͭ�Ͻ�
��1����ͭ��Cu2O�����ҹ��Ŵ���ȡ��ͭ��һ��ԭ�ϣ�Cu2O��ͭԪ������Ԫ�ص��������� ��
��2���������쵯�ǵĻ�ֻͭ����п��ͭ����22g���Ƿ���ʢ��100gϡ������ձ��У������������������Dz����ܽ���ձ��л�����������121.8g�����㣺
�ٲ���������������
�ڵ�����ͭ��������
�۷�Ӧ��������Һ��ZnSO4����������������������һλС������
��1����ͭ��Cu2O�����ҹ��Ŵ���ȡ��ͭ��һ��ԭ�ϣ�Cu2O��ͭԪ������Ԫ�ص���������
��2���������쵯�ǵĻ�ֻͭ����п��ͭ����22g���Ƿ���ʢ��100gϡ������ձ��У������������������Dz����ܽ���ձ��л�����������121.8g�����㣺
�ٲ���������������
�ڵ�����ͭ��������
�۷�Ӧ��������Һ��ZnSO4����������������������һλС������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���,Ԫ�������ȵļ���
ר�⣺�������������뻯ѧ����ʽ���ϵļ���,�йػ�ѧ����ʽ�ļ���,�й���Һ�������������ļ���
��������1�����ݳ�ͭ�Ļ�ѧʽ��Cu2O�����ɽ��
��2�������������غ㶨�ɿ��������ձ������ʵ�����֮���������������������
�ڸ����������������÷���ʽ���������Ӧ��п���������Ӷ��������������ͭ��������
�۸�������������Ҳ�������Ӧ���ɵ�����п���������������������Һ��ZnSO4������������
��2�������������غ㶨�ɿ��������ձ������ʵ�����֮���������������������
�ڸ����������������÷���ʽ���������Ӧ��п���������Ӷ��������������ͭ��������
�۸�������������Ҳ�������Ӧ���ɵ�����п���������������������Һ��ZnSO4������������
����⣺��1��Cu2O��ͭԪ������Ԫ�ص��������ǣ���64��2����16=8��1��
��2���ٻ�ͭ�е�ͭ�������ᷴӦ������������п����ϡ���ᷴӦ�������������������غ㶨�ɿ��������ձ������ʵ�����֮�������������������������ͼ�����ݿ�֪������Ϊ22g+100g-121.8g=0.2g��
���跴Ӧ��п��������x����������п��������y
Zn+H2SO4=ZnSO4+H2��
65 161 2
x y 0.2g
=
��
=
x=6.5g��y=16.1g
������ͭ��������22g-6.5g=15.5g��
�۷�Ӧ��������Һ��ZnSO4������������
��100%=15.1%��
�ʴ�Ϊ����1��8��1��
��2���ٲ���������������0.2g��
�ڵ�����ͭ��������15.5g��
�۷�Ӧ��������Һ��ZnSO4����������15.1%��
��2���ٻ�ͭ�е�ͭ�������ᷴӦ������������п����ϡ���ᷴӦ�������������������غ㶨�ɿ��������ձ������ʵ�����֮�������������������������ͼ�����ݿ�֪������Ϊ22g+100g-121.8g=0.2g��
���跴Ӧ��п��������x����������п��������y
Zn+H2SO4=ZnSO4+H2��
65 161 2
x y 0.2g
| 65 |
| x |
| 2 |
| 0.2g |
| 161 |
| y |
| 2 |
| 0.2g |
x=6.5g��y=16.1g
������ͭ��������22g-6.5g=15.5g��
�۷�Ӧ��������Һ��ZnSO4������������
| 16.1g |
| 121.8g-15.5g |
�ʴ�Ϊ����1��8��1��
��2���ٲ���������������0.2g��
�ڵ�����ͭ��������15.5g��
�۷�Ӧ��������Һ��ZnSO4����������15.1%��
�����������ѶȲ��Ǻܴ����������غ㶨���ɷ�Ӧǰ���ձ������ʵ��������IJ��������������������ǽ�����ͻ�ƿڣ�

��ϰ��ϵ�д�
 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
 ����֪ʶ������ѧϰ��ѧ����Ҫ����֮һ��ijͬѧ��ѧϰ�ᡢ����εĻ�ѧ���ʺ��ɵ�֪ʶ������ͼ��ͼ�С�-�����˵����ʿ��Է�Ӧ���������ͬѧ�������ƺ;�����
����֪ʶ������ѧϰ��ѧ����Ҫ����֮һ��ijͬѧ��ѧϰ�ᡢ����εĻ�ѧ���ʺ��ɵ�֪ʶ������ͼ��ͼ�С�-�����˵����ʿ��Է�Ӧ���������ͬѧ�������ƺ;����� 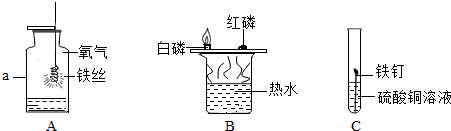



 ��Ϊ���л�ѧ�������ʣ���������������ش����⣮
��Ϊ���л�ѧ�������ʣ���������������ش����⣮