��Ŀ����
ij��ҵ���ס������������ŷŵķ�ˮ�к���K+��Cu2+�� Fe3+��C1-��OH-��NO3-�������ӣ��׳��������е����֣��ҳ������������֣�������ˮ��ֱ���ŷŶԵ���ˮ�ʴ�������Ӱ�졣ij����ʵ��С���������ˮ����ʵ�ؼ�⣬���ּ׳���ˮ�Լ��ԡ�
��1���׳���ˮ�п϶����е�������_____________,���ܺ��е�������_____________��
��2��������ʵ��С����ʵ�飬����������ˮ���ʵ�������ϣ��ɽ���ˮ�е�ijЩ����ת��Ϊ��������Щ�����ֱ���________________(�ѧʽ),���˺�ķ�ˮ����Ҫ����___________��������(�ѧʽ)��������ķ�ˮ�����ŷű���
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д���ȥ���������е��������ʣ���ѡ�õ��Լ��Ͳ�����������ȷ����
ѡ�� | ���� | ���� | �Լ� | �������� |
A | MnO2 | KCl | ����ˮ | �ܽ⡢���ˡ��������ᾧ |
B | CO2 | H2 | �������� | ��ȼ |
C | NaOH��Һ | Ca��OH��2 | ����Na2CO3��Һ | ���� |
D | ϡ���� | ���� | ������������Һ | ���� |
A.A B.B C.C D.D
�ܽ���ǽ����Һ����������Ҫ���ݡ�
I.�����ܽ�����߽����������: (M��N�������ᾧˮ)
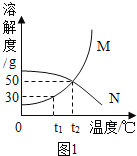
��1��t1��ʱ����20g M����50gˮ�У�����ܽ⣬�γ�_________(����͡������͡�)��Һ����Һ������Ϊ_____________g.�����¶Ȳ��䣬�����Һ���ټ���10gˮ��ֽ��裬��Һ����������������_______(��������С�����䡱) ;
��2��M�����к�������N���ʣ�����__________�����ᴿM����(����½ᾧ���������ᾧ��) ;
��3��t2��ʱ����25gN����50gˮ�У���ȫ�ܽ⡣������߸���Һ���������������������������____________��
II.���ݱ�����ʵ������:
�¶�/��C | 20 | 30 | 50 | 80 | 90 | |
�ܽ��/g | KNO3 | 31.6 | 45.8 | 85.5 | 100 | 169 |
K2CO3 | 110 | 114 | 121 | 126 | 139 |
ijKNO3��Ʒ�к�������K2CO3,���ᴿ������ͼ2(����������ˮ������û�б仯):
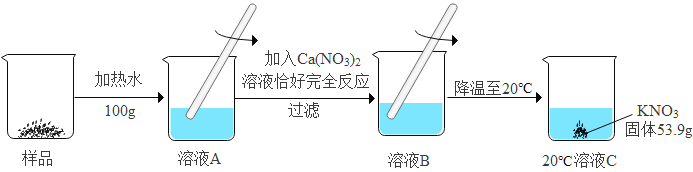
��1��ͼ2����ҺC��__________(����͡������͡�)��Һ; .
��2����Ʒ�м�Ԫ�ص�����Ϊ__________g (�����������).


 ��
��

