��Ŀ����
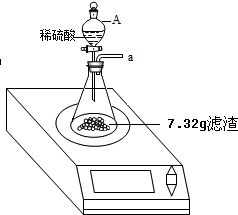
12��ijѧУ�о���ѧϰС��Ϊ�˲ⶨ���ؿ�ɽ��̼��Ƶ�����������ȡ����15g��ʯ��Ʒ��һ�����У���ȡϡ����200g��ƽ���ֳ�4�ݣ��ִμ������ʵ�飨ʯ��ʯ�е����ʲ�����ˮ�Ҳ����ᷢ����Ӧ������������¼���£��Լ��㣺| ����ϡ���������/g | 50 | 50 | 50 | 50 |
| ʣ����������/g | 11 | 7 | 5 | m |
��2��ʯ��ʯ��̼��Ƶ�����������
��3��ʵ��������ϡ�������������������
���� �ɱ������ݿ�֪��50gϡ�����ܺ�4g̼�����ȫ��Ӧ�������μ���50gϡ����ʱ����Ӧ��̼���������2g��˵�������μ���ϡ����ʱ��̼����Ѿ���ȫ��Ӧ������ϡ���������
��� �⣺��1����Ϊ�������е�̼����Ѿ���ȫ��Ӧ����˵��Ĵ��еĹ��岻�ټ��٣�m=5����
���5��
��2��ʯ��ʯ��̼��Ƶ���������Ϊ��$\frac{15g-5g}{15g}$��100%=66.7%��
��ʯ��ʯ��̼��Ƶ���������Ϊ66.7%��
��3����50gϡ�������Ȼ��������Ϊx��
50gϡ�����4g̼���ǡ����ȫ��Ӧ��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 73
4g x
$\frac{100}{4g}$=$\frac{73}{x}$��
x=2.92g��
ʵ��������ϡ�����������������Ϊ��$\frac{2.92g}{50g}$��100%=5.84%��
��ʵ��������ϡ�����������������Ϊ5.84%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
3������˵���������к������ǣ�������
| A�� | �����ᳫ����ĭ��ͺ� | |
| B�� | Ϊ�˷�ֹ����䣬�Դ����ļӵ��� | |
| C�� | ��������հ�װ���ٶ���ط�ֹ���ʸ��ñ��� | |
| D�� | ��������δ���е��ʵ�����������ĵ�����ˮ�ܵ� |
20���淶�IJ����ǻ�ѧʵ��ɹ��ı��ϣ�����ʵ�������ȷ���ǣ�������
| A�� | ��ȡ����ʱ�����÷���װ�ú����������� | |
| B�� | û�и�ʴ�ԵĹ���ҩƷֱ�ӷ��������ϳ��� | |
| C�� | �ⶨij���Լ���pHʱ������ֽ�������Һ�� | |
| D�� | ��ʵ����ʣ��ҩƷ��Ҫ��ʱ�Ż�ԭ�Լ�ƿ�� |
7����ȥ��������е��������ʣ���ѡ�õ��Լ���������������ȷ���ǣ�������
| ѡ�� | ���ʣ�������Ϊ���ʣ� | �Լ� | �������� |
| A | CO2��CO�� | ���� | ��ȼ |
| B | KCl��K2SO4�� | ����ˮ�����Ȼ�����Һ | �ܽ⡢���ˡ����� |
| C | NH3��H2O�� | ������Ũ���� | ϴ�� |
| D | Cu��CuO�� | ������ϡ���� | ���ݡ����ˡ�ϴ�ӡ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
2���������и������ʵ����ַ������������ǣ�������
| A | B | C | D | |
| ���ֵ����� | ��ͭ��18K�ƽ� | ��ۺ�̼� | �մ��С�մ� | ��Ȫˮ��ʳ��ˮ |
| ����һ | ����ϡ���� | ����ζ | ���� | ���� |
| ������ | �ڿ����м��� | �۲���ɫ | ��ζ�� | �ӷ���ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ij��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��
ij��ѧ��ȤС���ij�±���װ���еġ����������ܺ��棬���ǹ۲쵽�������������װ��ע�ijɷ�Ϊ���ۡ�����̿���Ȼ��ƣ����ֻҺ�ɫ�Ĺ����л���������������ɫ��ĩ��