��Ŀ����
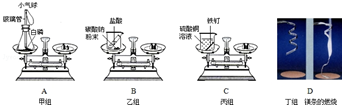
1����ͼ1��ʾ����������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
��1����ͼ1��ʾ����ű�ʾ������Һ����ȷ����˳��ڢݢ٢ܢۣ�
��2����������ƽ���������NaCIʱ������������ƽ��ָ��ƫ�����̣�ӦB����ѡ����ţ�
A����������NaCI���� B����������NaCI���� C������ƽ����ĸ
��3������NaCIʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ2�����ȡ��NaCI����Ϊ11.8g��
��4�����ݼ�����Ҫ��ȡˮ�������163.8��ˮ���ܶ�Ϊ1g/mL������ȡ����ʱ����ͼ3���߽Ƕ���ȷ����D����ѡ����ĸ��ţ�
��5��ͼ1�Т۵�Ŀ���ǽ��裬�����ܽ⣮
��6��������10%���Ȼ�����Һ������⣬������������ƫС����ԭ������Т٢ڢۢݣ�����ţ���
���Ȼ��ƹ��岻���� �ڳ���ʱ�����������������ͬ��ֽƬ
����ȡˮʱ�����Ӷ����� ��װƿʱ����������Һ������
�ݳ���NaCI������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�
���� ��1��������һ������������������Һ�IJ���ش�
��2��������������ƽ���������NaCIʱ������������ƽ��ָ��ƫ�����̣�Ӧ��������NaCI������
��3�������������=���������+�����������
��4�������ʵ���������������Һ���������ܼ�������=��Һ������-���ʵ�����������ˮ���ܶȼ����������������Ͳ��ȡҺ�����ʱ����Ҫ��Һ�尼Һ�����ʹ�����ˮƽ��
��5�����ݲ����������ý��
��6����������������С�����������������ƫС���ܼ�����ƫ���Է��������������������������ԭ����з������
��� �⣺��1������������������Ϊ10%��NaCl��Һ�IJ����ǣ����㡢��������ȡ���ܽ⣻
��2����������ƽ���������NaCIʱ������������ƽ��ָ��ƫ�����̣�Ӧ��������NaCI���壻
��3�����ݳ����������=���������+�����������֪����������ŷ������ȡ��NaCl����Ϊ��10g+5g-3.2g=11.8g��
��4��������Һ������Ϊ��$\frac{18.2g}{10%}$=182g��������Ҫˮ��������182g-18.2g=163.8g��ˮ���ܶ�Ϊ1g/mL������Ҫˮ�����Ϊ163.8mL����ȡ����ʱ�����߽Ƕ���ȷ����D��
��5��ͼ1�Т۵�Ŀ���ǽ��裬�����ܽ⣻
��6�������Ȼ��ƹ��岻���������ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС��
�ڳ���ʱ�����������������ͬ��ֽƬ�������ʵ����ȡ�����ʵ�����ƫ��С����ʹ������������ƫС��
����ȡˮʱ���Ӷ�����������ʵ��Һ�����С�������ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС��
����Һ���о�һ�ԣ�װƿʱ����������Һ���������������������䣮
�ݳ���NaCI������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ������ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС��
�ʴ�Ϊ����1���ڢݢ٢ܢۣ���2��ҩ�ף���3��11.8g����4��163.8mL�� D����5�����裬�����ܽ⣻��6���٢ڢۢݣ�
���� �˽Ⲣ������Ӧ��������Һ�����֪ʶ�������ʵ�������������Ͳ��ʹ�õ�֪ʶ������˳�����

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�| A�� | ������4.0��4.4 | B�� | ����2.2��2.4 | C�� | ������7.6��8.0 | D�� | ţ��6.3��6.6 |
| A�� | ���� | B�� | ֲ���� | C�� | ��ɳ | D�� | ��� |
��������衿��1�������ֽ����Ļ��˳����������ֿ��ܵļ��裺
a��Al��Cr��Cu b��Cr��Al��Cu c��Al��Cu��Cr
�����ʵ�顿ͬ���£�ȡ��С��ͬ�Ĵ�ĥ���Ľ�����Ƭ���ֱ�Ͷ�뵽�����Ũ�ȵ�����ϡ�����У��۲�����¼���£�
| ���� | Cr | Al | Cu |
| �����ᷴӦ�������� | ���ݲ����������������ܽ� | ���ݲ������ң�����Ѹ���ܽ� | �����ݲ����������ޱ仯 |
���ó����ۡ���3��ԭ��������ȷ����a���������a��b��c����д�������������ᷴӦ����+2�ۣ������ᷴӦ�Ļ�ѧ����ʽCr+2HCl�TCrCl2+H2����
��1����������⡿Al2O3�ܲ�������������ֽ�Ĵ�����
��2�����������롿Al2O3������������ֽ�Ĵ�����
��3����ʵ����֤��
| ʵ�鲽�� | ʵ������ | ʵ����� | |
| ʵ��һ | �������ǵ�ľ������װ�й���������Һ���Թ��� | ���������ݲ�����ľ������ȼ | �����¹���������Һ�ֽ����ʺ��� |
| ʵ��� | ��װ�й���������Һ���Թ��м�������Al2O3 ����Ȼ�����ǵ�ľ�������Թ��� | �д������ݲ�����ľ����ȼ | ����Al2O3���ܼӿ����������Һ�ķֽ����� |
��5������˼��ߡ��������ۣ��е�ͬѧ��Ϊֻ����������֤�ݣ�����֤��Al2O3������������ֽ�Ĵ�������Ҫ����һ��̽��ʵ������
ʵ������
��ʵ�鲽�衿��ȷ����Al2O3��������������ˮ���������������ʵ������۴���Ӧ��������ʵ����Թ�������ʽ��й��ˣ�ϴ�ӣ�����������ܶԱȷ�Ӧǰ��Al2O3��������
���������ۡ����Al2O3�ڷ�Ӧǰ���������䣬��˵��Al2O3��������������ֽ�Ĵ�����
������µ����⡿��С����Ϊ��Ҫ֤�����룬��������ʵ�黹���㹻������Ҫ������һ��̽��ʵ�飬�䷽������װ�й���������Һ���Թ��У�������˺�õ��Ĺ��壬�۲�����ͨ������ʵ�飬֤��Al2O3������������ֽ�Ĵ�����д��H2O2���ֽ�ķ���ʽ2H2O2 $\frac{\underline{\;Al_{2}O_{3}\;}}{\;}$2H2O+O2������