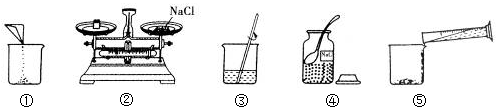
��Ŀ����
10����ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ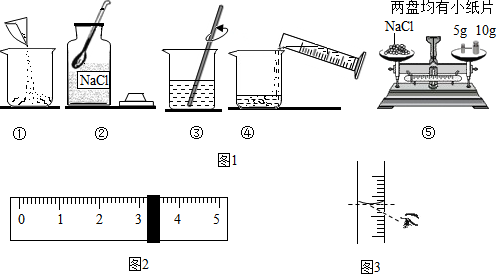
��1����ͼ1��ʾ����ű�ʾ������Һ����ȷ����˳��ڢݢ٢ܢۣ�
��2������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ2�����ȡ��NaCl������Ϊ18.2g��
��3�������������������ȷ��ֻ������ȡˮ�����ʱ������ͼ3��ʾ��ʽ����������������Һ�����ʵ���������С��10% ������ڡ��������ڡ���С�ڡ�����
���� ��1����������������������һ������Һ�Ļ������裬���з������
��2������������ƽ��ʹ�÷��������з������
��3������ͼ3��ʾ��ʽ����������Һ�棬������ʵ��Һ�����С�����з������
��� �⣺��1������������������Ϊ10%��NaCl��Һ�����ȼ���������Һ����NaCl��ˮ���������ٳ��������NaCl����ȡˮ���������ܽ⣬����ȷ����˳���Ǣڢݢ٢ܢۣ�
��2����NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ2�����ȡ��NaCl����Ϊ10g+5g+3.2g=18.2g��
��3������ͼ3��ʾ��ʽ����������Һ�棬������ʵ��Һ�����С�������ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС��
�ʴ�Ϊ����1���ڢݢ٢ܢۣ���2��18.2����3��С�ڣ�
���� �����ѶȲ�����ȷ����һ������������������Һʵ�鲽�裨���㡢�������ܽ⣩��ע�����������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
20�����л�ѧ����ʽ��д��ȷ���������û���Ӧ���ǣ�������
| A�� | CuSO4+BaCl2�TBaSO4��+CuCl2 | B�� | C+CuO�TCu+CO2�� | ||
| C�� | Cu+2AgNO3=2Ag+Cu��NO3��2 | D�� | 2KMnO4$\frac{\underline{\;����\;}}{\;}$K2MnO4+MnO2+O2�� |