��Ŀ����
ʵ���ǻ�ѧѧ�ƽ��п�ѧ̽������Ҫ;�����������ػ�Ӱ�컯ѧ��Ӧ�Ľ��У�
��1������������������Ӧ�������������ף������������������������ף����о���ѧ����ͬʵ�� ��
��2��31g����������Ӧ���������������������� ��������������Ӧ���������������������� ��
��3����31g����30g������Ӧʱ������Ϊ ��
��4��CO2��NaOH�ķ�Ӧ��������Ӧ���ƣ�CO2����ʱ��Ӧ���£�CO2+NaOH�TNaHCO3
CO2������ʱ��Ӧ���£�CO2+2NaOH�TNa2CO3+H2O����ͨ��CO2ʱ��Ӧ���̿ɿ������½��У��ȷ����˷�ӦCO2+2NaOH�TNa2CO3+H2O��
��CO2��ʣ�࣬��������Na2CO3+H2O+CO2�T2NaHCO3������80g��������Ϊ10%��NaOH��Һ��������ͨ��6.6gCO2�����������Na2CO3��NaHCO3��������
��1������������������Ӧ�������������ף������������������������ף����о���ѧ����ͬʵ��
��2��31g����������Ӧ����������������������
��3����31g����30g������Ӧʱ������Ϊ
��4��CO2��NaOH�ķ�Ӧ��������Ӧ���ƣ�CO2����ʱ��Ӧ���£�CO2+NaOH�TNaHCO3
CO2������ʱ��Ӧ���£�CO2+2NaOH�TNa2CO3+H2O����ͨ��CO2ʱ��Ӧ���̿ɿ������½��У��ȷ����˷�ӦCO2+2NaOH�TNa2CO3+H2O��
��CO2��ʣ�࣬��������Na2CO3+H2O+CO2�T2NaHCO3������80g��������Ϊ10%��NaOH��Һ��������ͨ��6.6gCO2�����������Na2CO3��NaHCO3��������
���㣺Ӱ�컯ѧ��Ӧ���ʵ�����̽��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���,��ѧ̽��
��������1���������������㣬̼��������Ӧ����һ����̼����������̼��������Ӧ���ɶ�����̼���
��2���������������Ļ�ѧʽ���������Ԫ�ص�����������Ȼ����������غ㶨��������������������Ϊ31g����Ԫ����������Ԫ�����������������ɵ��������������������������غ㶨�����ĵ�������������������������ȥ�ף�ͬ���ķ����������������������ʱ��Ҫ�������������
��3������������ȼ������������������������Ӧ��������������ʱ�������������Ƚ��
��4�����ݻ�ѧ����ʽ���м��㼴�ɣ�
��2���������������Ļ�ѧʽ���������Ԫ�ص�����������Ȼ����������غ㶨��������������������Ϊ31g����Ԫ����������Ԫ�����������������ɵ��������������������������غ㶨�����ĵ�������������������������ȥ�ף�ͬ���ķ����������������������ʱ��Ҫ�������������
��3������������ȼ������������������������Ӧ��������������ʱ�������������Ƚ��
��4�����ݻ�ѧ����ʽ���м��㼴�ɣ�
�����1����������̼��������ȼ�����ɶ�����̼����������̼��������ȼ������һ����̼����һ�������������ȼ�����ƣ��ʴ𰸣�̼����������ʱȼ�����ɶ�����̼����������ʱ����һ����̼��
��2��P2O3����Ԫ�ص���������Ϊ
=
�����ɵ���������������Ԫ�ص�����Ϊ31g���������ɵ���������������Ϊ
=55g��������Ҫ����������Ϊ55g-31g=24g��P2O5����Ԫ�ص���������Ϊ
=
�����ɵ���������������Ԫ�ص�����Ϊ31g�������ɵ���������������Ϊ
=71g��������������������Ϊ71g-31g=40g���ʴ𰸣�24g�� 40g
��3��4P+3O2
2P2O3����������Ӧ����������124��96=31��24��4P+5O2
2P��2O5������������������124��160=31��40�����Ե�31g����30g������Ӧʱ������Ϊ�������������������Ļ����ʴ𰸣��������������������Ļ����
��4���⣺�����ȷ�Ӧ��̼���Ƶ�����Ϊx�����Ķ�����̼y��
CO2+2NaOH�TNa2CO3+H2O��
44 2��40 106
y 80g��10% x
=
��ã�x=10.6g y=4.4g
ʣ�������̼������Ϊ��6.6g-4.4g=2.2g
�跴Ӧ����NaHCO3����Ϊa������Na2CO3����Ϊb��
Na2CO3+H2O+CO2�T2NaHCO3
106 44 2��84
b 2.2g a
=
��ã�a=8.4g b=5.3g
��ʣ��Na2CO3����Ϊ��10.6g-5.3g=5.3g
������Na2CO3����Ϊ5.3g������NaHCO3����Ϊ8.4g��
��2��P2O3����Ԫ�ص���������Ϊ
| 31��2 |
| 31��2+16��3 |
| 62 |
| 110 |
| 31g | ||
|
| 62 |
| 62+80 |
| 62 |
| 142 |
| 31g | ||
|
��3��4P+3O2
| ||
| ||
��4���⣺�����ȷ�Ӧ��̼���Ƶ�����Ϊx�����Ķ�����̼y��
CO2+2NaOH�TNa2CO3+H2O��
44 2��40 106
y 80g��10% x
| 44 |
| 2��40 |
| y |
| 80g��10% |
| 2��40 |
| 106 |
| 80g��10% |
| x |
��ã�x=10.6g y=4.4g
ʣ�������̼������Ϊ��6.6g-4.4g=2.2g
�跴Ӧ����NaHCO3����Ϊa������Na2CO3����Ϊb��
Na2CO3+H2O+CO2�T2NaHCO3
106 44 2��84
b 2.2g a
| 106 |
| b |
| b |
| 2.2g |
| 44 |
| 2��84 |
| 2.2g |
| a |
��ã�a=8.4g b=5.3g
��ʣ��Na2CO3����Ϊ��10.6g-5.3g=5.3g
������Na2CO3����Ϊ5.3g������NaHCO3����Ϊ8.4g��
��������������֪ʶ�����龰���Ƶ�����£�����֪ʶ�ش�Ǩ�ƣ��ǽ���������һ����Ҫ���������ݻ�ѧ����ʽ�ļ����ʽ����ȷ���ղ��ܼ���ɹ���

��ϰ��ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A��ͬһ���ʵ���Һ����һ���¶��£�������Һ�Ȳ�������ҺŨ�ȴ� |
| B�����ʵ��ܽ�����¶ȵ����߶����� |
| C�������¶ȣ��κ����ʵı�����Һ�����Ա�Ϊ��������Һ |
| D��10��ʱ��50gˮ��������ܽ�40g�����ƣ�����ͬ�¶��£�100gˮ��������ܽ�80g�����ƣ���˵�������ܼ��������ӣ����ʵ��ܽ��Ҳ���� |
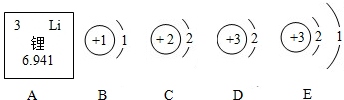
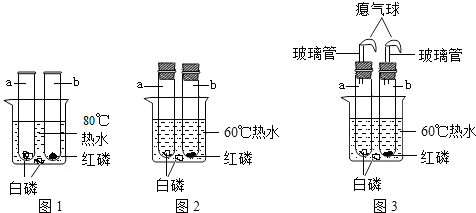
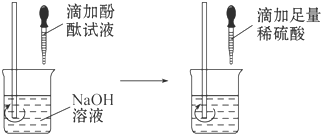
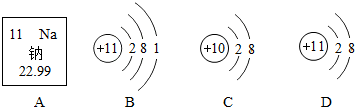 ��ͼ�ش�
��ͼ�ش�