��Ŀ����
3��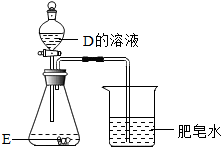 ������ĸA��H�������л��г��������ʣ��������⡢̼�������ơ��ơ����е�2-3��Ԫ�����
������ĸA��H�������л��г��������ʣ��������⡢̼�������ơ��ơ����е�2-3��Ԫ�������1��A��ǿ�ҵĸ�ʴ�ԣ��׳��⣬��ɫ��̪��Һ��A��ϡ��Һ��죬A���׳��ǻ��ռ�����ƣ�
��2����ɫ��ĩB������C��Ӧ�õ���ɫ��ĩ����ѧ����ʽΪFe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2
��3��D��E��������Ԫ�أ���ͼ��ʾ����D����Һ������ƿ�У���Һ��ƣ������ݲ������ձ��оۼ��ķ������ܱ���ȼ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪFe+2HCl�TFeCl2+H2����Fe2O3+6HCl�T2FeCl3+3H2O
��4��F��G��H�ֱ���D����Һ��Ӧ������ˮ���ɣ���F����Һ��G����Һ��ϣ����ɰ�ɫ����H��F��G��Ӧ�Ļ�ѧ����ʽΪCa��OH��2+Na2CO3�TCaCO3��+2NaOH��
���� ��1������A�������׳��⣬ϡҺ�ʼ��ԣ����ƶϳ�A��NaOH��
��2����ɫ��ĩ��������������������һ����̼��̼�ȷ�Ӧ��
��3������Һ��ƿ�֪��Ӧ���к�����Ԫ�أ������ݲ����ҿ�ȼ�ó�������������˵����Ӧ���к�����Ԫ�أ�
��4������������D������Һ��Ӧ����ˮ���ɿ�֪����Ӧ��Ӧ���Ǽ��̼���Σ����ɽ�F����Һ��G����Һ������ɰ�ɫ����H��֪��H��̼��ƣ������ó�F��G���������ƺ�̼���ƣ�
��� �⣺��1������A�������׳��⣬ϡҺ�ʼ��ԣ����ƶϳ�A��NaOH��
��2����ɫ��ĩ��������������һ����̼��Ӧ��Ӧ���ɵ��ʵ����Ͷ�����̼��ע��C��������������Ϊ��ĿҪ����2-3��Ԫ����ɣ�
��3������Һ��ƿ�֪��Ӧ���к�����Ԫ���ҷ������Ǹ��ֽⷴӦ�������ݲ����ҿ�ȼ���ж������������ҷ��������û���Ӧ��˵����Ӧ���к�����Ԫ�أ��ʿ��ƶ������������Dϡ���ᷴӦ��
��4������������D������Һ��Ӧ����ˮ���ɿ�֪����Ӧ��Ӧ���Ǽ��̼���Σ�F��G��H�ж�����OԪ�أ����ɽ�F����Һ��G����Һ������ɰ�ɫ����H��֪��H��̼��ƣ������ó�F��G���������ƺ�̼���ƣ�
�ʴ�Ϊ��
��1�����ռ�����ƣ���
��2��Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
��3��Fe+2HCl�TFeCl2+H2����Fe2O3+6HCl�T2FeCl3+3H2O��
��4��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
���� ������ƶ���������ʵ�Ԫ����Ϊ����������ˣ���Ϥ�������ʵ���ɡ����ʼ��仯���ɶԸ��������漰���ʵ��ƶϾ�������Ҫ�ˣ�

| A�� | ����������ȼ�շ�������ɫ���� | |
| B�� | ȼ��һ����Ҫ�����μ� | |
| C�� | �����ľƾ�������ȼ�գ���ʪ������ | |
| D�� | ������ȥ�����������黯���� |
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ����H2��CH4 | �ֱ��ȼ�����ڻ����Ϸ��ָ����ձ� |
| B | ����һƿ�����Ƿ�ΪCO2 | ��ȼ�ŵ�ľ������ƿ�� |
| C | �������͵�ʯ��ˮ�䱥�� | ���������������� |
| D | ��ȥ���ֱ����ռ��е�̼���� | ��������ʯ��ˮ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
��1����ʯȼ����ú��ʯ�ͺ���Ȼ������ʯȼ�����ڲ��������������������������������Դ
��2��������ȫȼ�յĻ�ѧ����ʽΪCH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O
��3���ӱ������ݷ�������ú��ȣ�����Ȼ����ȼ�ϵ��ŵ��Dz����Ķ�����̼�٣��ų��������ࣨ��1�㼴�ɣ�
| 1g������ȫȼ�ղ��� | |||
| �ų�������/kJ | CO2������/g | ����SO2������/mg | |
| ���� | 56 | 2.75 | 0.3 |
| ú | 32 | 3.67 | 11 |
| A�� |  ˮ��ե��֭ | B�� |  �ƶ����� | C�� |  Ŵ������� | D�� |  ���������� |



