��Ŀ����
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
��1��������Ʒ��ȫ��Ӧ���������_________g��
��2���û��ʵĺ�����Ϊ_________����ȷ��0.1%��������_________��ѡ��ϸ��ϸ���Ʒ��
��3�������������հ�������Һ�е���������������Ƕ��٣�д��������̣���ȷ��0.1%����
��1��������Ʒ��ȫ��Ӧ���������_________g��
��2���û��ʵĺ�����Ϊ_________����ȷ��0.1%��������_________��ѡ��ϸ��ϸ���Ʒ��
��3�������������հ�������Һ�е���������������Ƕ��٣�д��������̣���ȷ��0.1%����

��1��6.8
��2��20.4%���ϸ�
��3������Һ����������淋�����Ϊx
2NH3+H2SO4=��NH4��2SO4
34 ����������132
6.8g ����������x
 =
=
x=26.4g
��Һ������淋�������������Ϊ ��100%=24.7%
��100%=24.7%
����Һ������淋�������������Ϊ24.7%
��2��20.4%���ϸ�
��3������Һ����������淋�����Ϊx
2NH3+H2SO4=��NH4��2SO4
34 ����������132
6.8g ����������x
 =
=
x=26.4g
��Һ������淋�������������Ϊ
 ��100%=24.7%
��100%=24.7%����Һ������淋�������������Ϊ24.7%

��ϰ��ϵ�д�
 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
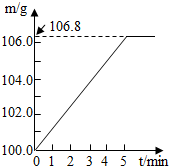 ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к��������ͣ�20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺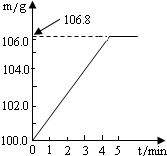
 ��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ���������
��2011?��ƽ����ģ��ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�����ȡ27.5g������Ʒ������Ũ�ռ���Һ���ȣ������ĵ�����100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯��ͼ��ʾ�����漰�ķ�ӦΪ����NH4��SO4+2NaOH=Na2SO4+2NH3+2H2O����2NH3+H2SO4=��NH4��SO4������㣺��1����ȫ��Ӧ��������� ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺
ijͬѧΪ�˼�����ﹺ�������炙����Ƿ�ϸ�ȡ��27.5g������Ʒ������Ũ�ռ���Һһ����ȣ������İ�����������100.0g���������գ�������հ�������Һ������m�뷴Ӧʱ��t�ı仯����ͼ��ʾ����֪�ϸ�����炙����к�����������20%�����漰�ķ�ӦΪ��NH4��2SO4+2NaOH=Na2SO4+2H2O+2NH3����2NH3+H2SO4=��NH4��2SO4���Լ��㣺