��Ŀ����
����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���
�Իش��������⣺
��1����������ֽ�ϣ��Է�Ӧʱ��Ϊ���ᣬ�Բ���������̼���������Ϊ���ᣬ������������������淴Ӧʱ��仯�Ĺ�ϵ���ߣ�
��2���ӱ��п��Կ�����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����______g��
��3����ʯ��ʯ��̼��Ƶ�������������д��������̣�
| ʱ��/s | 0 | 30 | 60 | 90 | 120 | 150 | 180 |
| ����/g | 0 | 15 | 25 | 30 | 33 | 33 | 33 |
��1����������ֽ�ϣ��Է�Ӧʱ��Ϊ���ᣬ�Բ���������̼���������Ϊ���ᣬ������������������淴Ӧʱ��仯�Ĺ�ϵ���ߣ�
��2���ӱ��п��Կ�����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����______g��
��3����ʯ��ʯ��̼��Ƶ�������������д��������̣�

��1�����ݲⶨ���ݱ��е�ʱ������������ȷ��һЩ�㣬Ȼ��Ѹ�������ƽ������ͼ���ӣ���������ʱҪ����������ƽ����������ȷ��
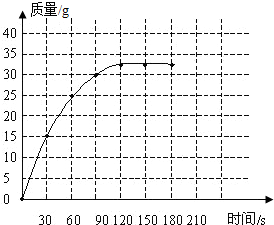
��2��������¼��ʵ�����ݿ�֪�������ն�����̼�����ֵΪ33g����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����33g��
��3����μӷ�Ӧ��̼��Ƶ�����Ϊx���μӷ�Ӧ��HCl������Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 33g
=
=
x=75g��y=54.75g
ʯ��ʯ��̼��Ƶ���������Ϊ��
��100%=75%��
��ʯ��ʯ��̼��Ƶ���������Ϊ75%
��1����ͼ

��2��33��
��3����ʯ��ʯ��̼��Ƶ���������Ϊ75%

��2��������¼��ʵ�����ݿ�֪�������ն�����̼�����ֵΪ33g����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����33g��
��3����μӷ�Ӧ��̼��Ƶ�����Ϊx���μӷ�Ӧ��HCl������Ϊy
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 33g
| 100 |
| x |
| 73 |
| y |
| 44 |
| 33g |
x=75g��y=54.75g
ʯ��ʯ��̼��Ƶ���������Ϊ��
| 75g |
| 100g |
��ʯ��ʯ��̼��Ƶ���������Ϊ75%
��1����ͼ

��2��33��
��3����ʯ��ʯ��̼��Ƶ���������Ϊ75%

��ϰ��ϵ�д�
�����Ŀ
����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���
�Իش��������⣺
��1����������ֽ�ϣ��Է�Ӧʱ��Ϊ���ᣬ�Բ���������̼���������Ϊ���ᣬ������������������淴Ӧʱ��仯�Ĺ�ϵ���ߣ�
��2���ӱ��п��Կ�����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����______g��
��3����ʯ��ʯ��̼��Ƶ�������������д��������̣�
| ʱ��/s | 30 | 60 | 90 | 120 | 150 | 180 | |
| ����/g | 15 | 25 | 30 | 33 | 33 | 33 |
��1����������ֽ�ϣ��Է�Ӧʱ��Ϊ���ᣬ�Բ���������̼���������Ϊ���ᣬ������������������淴Ӧʱ��仯�Ĺ�ϵ���ߣ�
��2���ӱ��п��Կ�����100gʯ��ʯ������ϡ���ᷴӦ���ɵĶ�����̼�����______g��
��3����ʯ��ʯ��̼��Ƶ�������������д��������̣�

 ����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���
����ʯ��ʯ��Դ�ḻ����Ϊ���з�չ���Ĺ�ҵ�ṩ�˵�����������������ijУ���꼶��ѧ�о���ѧϰС���ͬѧ���ڿ����̽����вɼ��˵���һ��ɽ�ϵ�ʯ��ʯ��Ʒ����ͨ��ʵ��ⶨʯ��ʯ��Ʒ��̼��Ƶĺ���������ȡ100gʯ��ʯ��Ʒ�����ʲ������ˮ��Ӧ�������ձ��в�����300gϡ���ᣬǡ����ȫ��Ӧ���Ѳ����Ķ�����̼������������ʯ��ˮ���գ�ͬʱ����3�������ձ������ص�������������±���