��Ŀ����
ijͬѧ������ͼ��1����ʾ��װ������֤��С���빳����ɵ�ϵͳ��е���غ�
��1�����ṩ�������ģ�
A��С�� B������ C��һ�˴����ֵij�ľ�� D��ϸ�� E��������� F��ֽ�� G�����̶ȳ� H���α꿨��
I����ѹ������Դ J��220V������Դ��
ʵ���в���Ҫ��������______����д����ǰ����ĸ��������Ҫ��������______
��2��Ϊ����������Ħ�����Ա�ʵ���Ӱ�죬ʹ��֤���������ȷ����С������M��������m�Ĺ�ϵӦ������______
��3��С����С��ͬѧ������ʵ��ʱ��С����ΪֻҪ����ƽ��Ħ�����ķ�����Ħ����ƽ����Ϳ����ˣ��������㣨2������
С������M��������m�Ĺ�ϵ����С��ȴ����С���Ŀ���������ΪС���Ŀ���______��ѡ���ȷ������ȷ������
������______
��4����λͬѧͳ���۵�����ʵ�飬�õ�һ��ֽ����ȥ��ǰ��Ƚ��ܼ��ĵ㣬rѡ��㼣�����ұ��ڲ���������7���㣨���
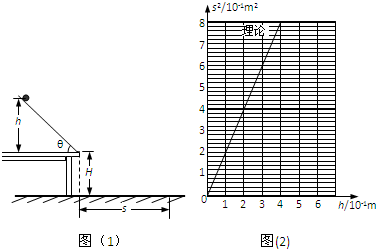
Ϊ0-6���������ؾ�����ͼ��2����ʾ��Ҫ��֤�ڵ�2�����5��ʱϵͳ�Ļ�е����ȣ���Ӧ�����ϵʽ______����С��
����M��������m���������ΪT����

��1�����ṩ�������ģ�
A��С�� B������ C��һ�˴����ֵij�ľ�� D��ϸ�� E��������� F��ֽ�� G�����̶ȳ� H���α꿨��
I����ѹ������Դ J��220V������Դ��
ʵ���в���Ҫ��������______����д����ǰ����ĸ��������Ҫ��������______
��2��Ϊ����������Ħ�����Ա�ʵ���Ӱ�죬ʹ��֤���������ȷ����С������M��������m�Ĺ�ϵӦ������______
��3��С����С��ͬѧ������ʵ��ʱ��С����ΪֻҪ����ƽ��Ħ�����ķ�����Ħ����ƽ����Ϳ����ˣ��������㣨2������
С������M��������m�Ĺ�ϵ����С��ȴ����С���Ŀ���������ΪС���Ŀ���______��ѡ���ȷ������ȷ������
������______
��4����λͬѧͳ���۵�����ʵ�飬�õ�һ��ֽ����ȥ��ǰ��Ƚ��ܼ��ĵ㣬rѡ��㼣�����ұ��ڲ���������7���㣨���
Ϊ0-6���������ؾ�����ͼ��2����ʾ��Ҫ��֤�ڵ�2�����5��ʱϵͳ�Ļ�е����ȣ���Ӧ�����ϵʽ______����С��
����M��������m���������ΪT����

��1��ʵ�鲻��Ҫ�α꿨�ߣ������ɿ̶ȳ߲��������ʱ��ֱ��ʹ��220V������Ҫ��ѹ������Դ��
����Ҫ��������H��I������Ҫ��ƽ���������������
��2��Ϊ����������Ħ�����Ա�ʵ���Ӱ�죬ʹ��֤���������ȷ����С������M��������m�Ĺ�ϵӦ������M����m��
������ʹĦ�������Ĺ������Щ���Լ�С��е�ܵ���ʧ��
��3��С���Ŀ�������ȷ����Ϊƽ��Ħ������Ħ��������������ϵͳ��е�ܲ��غ㣮
��4�������ȱ���ֱ���˶������ۣ�
v2=
��
v5=
��2�����5��ʱϵͳ�Ķ�������
��M+m����
��2-
��M+m����
��2
��2�����5��ʱϵͳ���������ܼ�С����mg��d5-d2��
Ӧ�����ϵʽmg��d5-d2��=
��M+m����
��2-
��M+m����
��2
�ʴ�Ϊ��
��1��H��I����ƽ
��2��M����m
��3������ȷ����Ϊƽ��Ħ������Ħ��������������ϵͳ��е�ܲ��غ㣮
��4��mg��d5-d2��=
��M+m����
��2-
��M+m����
��2
����Ҫ��������H��I������Ҫ��ƽ���������������
��2��Ϊ����������Ħ�����Ա�ʵ���Ӱ�죬ʹ��֤���������ȷ����С������M��������m�Ĺ�ϵӦ������M����m��
������ʹĦ�������Ĺ������Щ���Լ�С��е�ܵ���ʧ��
��3��С���Ŀ�������ȷ����Ϊƽ��Ħ������Ħ��������������ϵͳ��е�ܲ��غ㣮
��4�������ȱ���ֱ���˶������ۣ�
v2=
| d3-d1 |
| 2T |
v5=
| d6-d4 |
| 2T |
��2�����5��ʱϵͳ�Ķ�������
| 1 |
| 2 |
| d6-d4 |
| 2T |
| 1 |
| 2 |
| d3-d1 |
| 2T |
��2�����5��ʱϵͳ���������ܼ�С����mg��d5-d2��
Ӧ�����ϵʽmg��d5-d2��=
| 1 |
| 2 |
| d6-d4 |
| 2T |
| 1 |
| 2 |
| d3-d1 |
| 2T |
�ʴ�Ϊ��
��1��H��I����ƽ
��2��M����m
��3������ȷ����Ϊƽ��Ħ������Ħ��������������ϵͳ��е�ܲ��غ㣮
��4��mg��d5-d2��=
| 1 |
| 2 |
| d6-d4 |
| 2T |
| 1 |
| 2 |
| d3-d1 |
| 2T |

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ijͬѧ������ͼ��1����ʾ��·�ⶨ��Դ�ĵ綯�ƺ����裮ʵ���е�������ʵ������Ӱ���С�������Ժ��Բ��ƣ��պϵ��S�������Ļ�ƬP�ɱ�������һ�˻�����һ�˵Ĺ����У�����ѹ��ʾ���������ʾ���仯����ֱ���ͼ��2����U-Iͼ����ֱ��a��b��ʾ��
ijͬѧ������ͼ��1����ʾ��·�ⶨ��Դ�ĵ綯�ƺ����裮ʵ���е�������ʵ������Ӱ���С�������Ժ��Բ��ƣ��պϵ��S�������Ļ�ƬP�ɱ�������һ�˻�����һ�˵Ĺ����У�����ѹ��ʾ���������ʾ���仯����ֱ���ͼ��2����U-Iͼ����ֱ��a��b��ʾ��