��Ŀ����
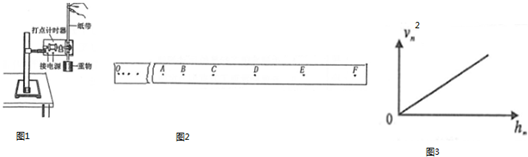
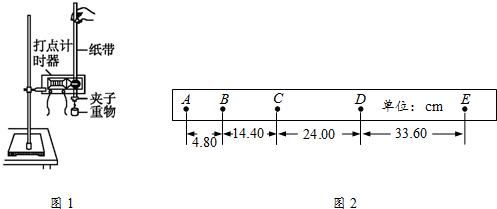
10����ͼ����ʾ���ó�ֱ���졢����š����ּ�ʱ����װ������֤ţ�ٵڶ�����ʵ�飬��ش��������⣺
��1��ƽ��Ħ������Ҫ�������ڸߡ��͵���ťʹ��ֱ������һ������ǣ��ڲ��ҹ���ʱ����С��һ�£��۲쵽С�������»��ĸ����Ǿ�����������ŵĵ���ʱ����ȣ�
��2�����Ϲ���ʱҪ���ں�С���ֵĸ߶�ʹ����С����ϸ���볤ֱ����ƽ�У�
��3��������������������Ƭ�ĺ��ʱʾ����ͼ����ʾ������Ϊ1.510mm��
���� ��1��ƽ��Ħ����ʱ�����ܽ�����ͨ�������ֹ���С���ϣ���С������ֽ���˶�������С������С��������ֱ���˶���Ħ�����õ�ƽ�⣮
��2�����Ϲ���ʱҪ���ں�С���ֵĸ߶�ʹ����С����ϸ�����볤ֱ����ƽ�У�
��3�����������Ķ��������ǹ̶��̶ȶ������Ͽɶ��̶ȶ������ڶ��ɶ��̶ȶ���ʱ�������
��� �⣺��1��С�������»�ʱ�ٶȲ��䣬��������ŵĵ���ʱ����ȣ�
��2����װ�ڳ�ֱ����һ�˵�С���ָ߶ȿ��Ե��ڣ�ǣ��С����ϸ��Ӧ�볤ֱ����ƽ�У��Ա�֤���Ӷ�С���������������ӵ�������
��3���ӹ̶��̶ȴ��Թ���������ӦΪ1.5mm���ң��ɼ����߸պ�¶������ȷ����Ϊ1.510mm��
�ʴ�Ϊ����1��������������ŵĵ���ʱ����ȡ���2����ֱ����ƽ�С���3��1.510
���� �������Ĺؼ�����ƽ��Ħ�����ķ������Լ�����ֽ���Ĵ�������ͨ��ֽ�����˲ʱ�ٶȺͼ��ٶȣ��ؼ����ȱ���ֱ���˶����۵����ã�

��ϰ��ϵ�д�
�����Ŀ
18��ѧϰ��������֪ʶ��ѧϰ�⣬��Ҫ�������մ������������˼���뷽��������ͼʾ������ѧϰ���ļ�������ʵ�飬���з�����ͬ������ʵ��ʱ��������
| A�� |  ����̽��Ӱ�쵼���������� ����̽��Ӱ�쵼���������� | B�� | 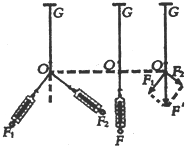 ̽��������ķ��� | ||
| C�� |  ̽�����ٶ������������Ĺ�ϵ | D�� |  �۲�����С�α� |
5�� ��ͼ��ʾ��Ϊ��ͬһ���������ļס������м�г�Შijʱ�̵IJ���ͼ������a��b��c�Ǽײ��ϵ������㣮����˵����ȷ���ǣ���������
��ͼ��ʾ��Ϊ��ͬһ���������ļס������м�г�Შijʱ�̵IJ���ͼ������a��b��c�Ǽײ��ϵ������㣮����˵����ȷ���ǣ���������
 ��ͼ��ʾ��Ϊ��ͬһ���������ļס������м�г�Შijʱ�̵IJ���ͼ������a��b��c�Ǽײ��ϵ������㣮����˵����ȷ���ǣ���������
��ͼ��ʾ��Ϊ��ͬһ���������ļס������м�г�Შijʱ�̵IJ���ͼ������a��b��c�Ǽײ��ϵ������㣮����˵����ȷ���ǣ���������| A�� | �����в�����ʱ�ܷ����������� | |
| B�� | �ײ����ٶ�v�����Ҳ����ٶ�v���� | |
| C�� | �ʵ�a���ʵ�b�Ȼص�ƽ��λ�� | |
| D�� | ��v��=20m/s���پ�t=0.5s���ʵ�c�˶���·���� 0.5m |
15�������ȼ���ֱ���˶�������˵����ȷ���ǣ�������
| A�� | λ����ʱ���ƽ�������� | |
| B�� | λ��������ʱ�����Ӷ����� | |
| C�� | ���ٶȡ��ٶȡ�λ�����߷���һ�� | |
| D�� | ���ٶȡ��ٶȡ�λ�Ƶķ����Ƕ���ͬ |
2��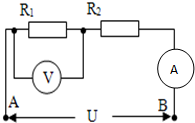 ��ͼ��ʾ�ĵ�·�У�����R1��R2��������һ�㶨��ѹUAB=5V�ĵ�Դ���ˣ���������֪R1���˵�ѹU1=2V������������Ϊ1A����R2�ĵ��裨������
��ͼ��ʾ�ĵ�·�У�����R1��R2��������һ�㶨��ѹUAB=5V�ĵ�Դ���ˣ���������֪R1���˵�ѹU1=2V������������Ϊ1A����R2�ĵ��裨������
 ��ͼ��ʾ�ĵ�·�У�����R1��R2��������һ�㶨��ѹUAB=5V�ĵ�Դ���ˣ���������֪R1���˵�ѹU1=2V������������Ϊ1A����R2�ĵ��裨������
��ͼ��ʾ�ĵ�·�У�����R1��R2��������һ�㶨��ѹUAB=5V�ĵ�Դ���ˣ���������֪R1���˵�ѹU1=2V������������Ϊ1A����R2�ĵ��裨������| A�� | 2�� | B�� | 3�� | C�� | 5�� | D�� | 7�� |
19��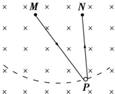 ��ͼ��ʾ���ռ����д�ֱֽ���������ǿ�ų���һ�����������������ƹ�ֽ���ڵ�С������P������Ϊ�ʵ㣩�����˷ֱ�˩��ֽ���ڵ������̶���M��N������ͨ����M��N�ĺ㶨����I������PM��PNʼ����ֱ���ֽ�P�������ƶ����Ҳ࣬�ڴ˹����е���MPN�ܵ��İ�������С��������
��ͼ��ʾ���ռ����д�ֱֽ���������ǿ�ų���һ�����������������ƹ�ֽ���ڵ�С������P������Ϊ�ʵ㣩�����˷ֱ�˩��ֽ���ڵ������̶���M��N������ͨ����M��N�ĺ㶨����I������PM��PNʼ����ֱ���ֽ�P�������ƶ����Ҳ࣬�ڴ˹����е���MPN�ܵ��İ�������С��������
 ��ͼ��ʾ���ռ����д�ֱֽ���������ǿ�ų���һ�����������������ƹ�ֽ���ڵ�С������P������Ϊ�ʵ㣩�����˷ֱ�˩��ֽ���ڵ������̶���M��N������ͨ����M��N�ĺ㶨����I������PM��PNʼ����ֱ���ֽ�P�������ƶ����Ҳ࣬�ڴ˹����е���MPN�ܵ��İ�������С��������
��ͼ��ʾ���ռ����д�ֱֽ���������ǿ�ų���һ�����������������ƹ�ֽ���ڵ�С������P������Ϊ�ʵ㣩�����˷ֱ�˩��ֽ���ڵ������̶���M��N������ͨ����M��N�ĺ㶨����I������PM��PNʼ����ֱ���ֽ�P�������ƶ����Ҳ࣬�ڴ˹����е���MPN�ܵ��İ�������С��������| A�� | ʼ�ղ��� | B�� | ������ | C�� | ��������С | D�� | �ȼ�С������ |
20����ͼΪ���糡��һ���ֵ糡�ߵķֲ�������˵����ȷ���ǣ�������
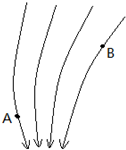

| A�� | ����糡�����Ǹ����ɵĵ糡 | |
| B�� | ����糡��������ǿ�糡 | |
| C�� | ������A���ܵ��ĵ糡������B��ʱ�ܵ��ĵ糡���� | |
| D�� | �������B��ʱ�ܵ��ĵ糡���ķ�����B�����߷��� |