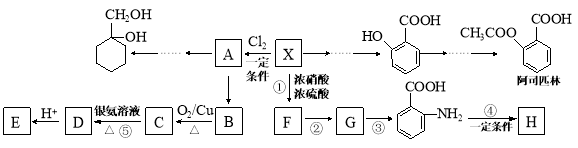
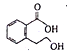
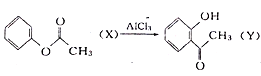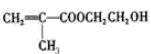��Ŀ����
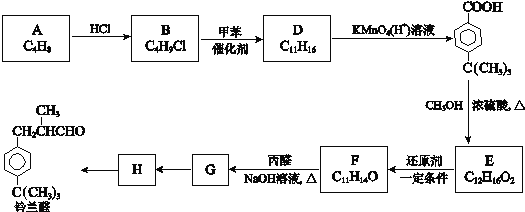
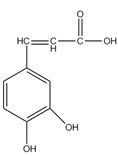
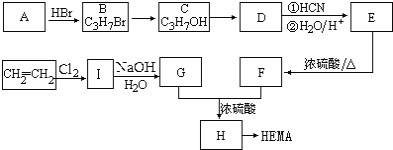
����Ŀ�����������ű������ڡ��ƹ������м�������ܻ����������ܻ��������������ܼ�����ѧ�����ڱ��������������������ѧʽ��C16H22O4 , ���ڹ�ҵ����;�dz��㷺������������������Σ�����������ܻ���������ܶ࣬����һ�֡��ܻ��������Ʊ�������ͼ��
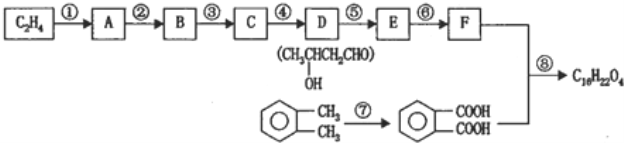
��֪��
��ش��������}��
��1������ˮ�ⷴӦ���䷴Ӧ����Ϊ�� ��
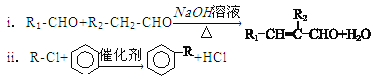
��2��д����ͼ��Ӧ�ķ�Ӧ���ͣ��� ���� ���� ��
��3��д�����з���ʽ���� ���� ���� ��
��4��д��D�����������Һ�����������ͬ���칹���� �֣�д������һ�ֺ˴Ź���������3���壬����ԭ����Ϊ6��1��1�Ľṹʽ ��
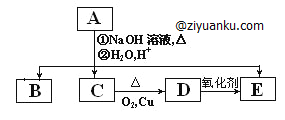
��������1����������ˮ��Һ���� ��2���ӳɷ�Ӧ����ȥ��Ӧ��������Ӧ��
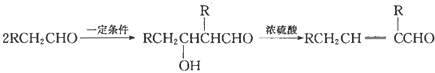
��3����2CH3CH2OH+O2![]() 2CH3CHO+2H2O����CH3CH��CHCHO+2H2
2CH3CHO+2H2O����CH3CH��CHCHO+2H2![]() CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��
��![]()
��4��3��HCOOCH��CH3��2
��������
�����������D�Ľṹ��ʽ�Լ���֪��Ϣ��֪CΪCH3CHO����BΪCH3CH2OH��AӦΪCH3CH2Br��CH3CH2Cl��EΪCH3CH��CHCHO��FΪCH3CH2CH2CH2OH����![]() ����������Ӧ�����ɷ���ʽΪC18H22O4��������
����������Ӧ�����ɷ���ʽΪC18H22O4��������
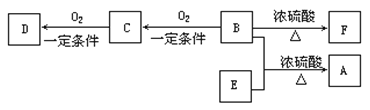
��1������±������ˮ�ⷴӦ���䷴Ӧ����Ϊ�������Ƶ�ˮ��Һ���ȡ�
��2�������Ϸ�����Ϲ����ŵı仯��֪��Ϊ��ϩ��HX���ӳɷ�Ӧ����Ϊ�ǻ�����ȥ��Ӧ����Ϊ�����ϼ���������Ӧ��
��3����Ϊ�Ҵ�������������Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��ΪCH3CH��CHCHO���������ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CH��CHCHO+2H2![]() CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��
��ΪCH3CH2CH2CH2OH��![]() ������������Ӧ����Ӧ�Ļ�ѧ����ʽΪ
������������Ӧ����Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
��4��D�ķ���ʽΪC4H8O2����Ӧ�����������Һ�����������ͬ���칹����CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH��CH3��CH3����3�֣�����һ�ֺ˴Ź���������3���壬����ԭ����Ϊ6��1��1�ĽṹʽΪHCOOCH��CH3��2��

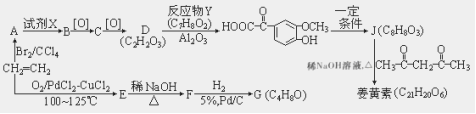
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�����Ŀ����������ͼ��ʾװ�ý���ʵ�飬�����٢ڢ���װ�����Լ�����������������ȷ����

ѡ�� | �� | �� | �� | �Թܢ������� |
A | Ũ���� | Na2SO3 | Ba(NO3)2��Һ | ������ |
B | ϡ���� | ����ʯ | ��������Һ | ������ɫ���� |
C | ϡ���� | ����ʯ | CaCl2��Һ | ������ɫ���� |
D | Ũ���� | ͭƬ | KI��������Һ | ��Һ���� |