��Ŀ����
6��ij����ɫ������A�ڳ��º���������£������ȶ����ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������ͬʱ����һ�����嵥�ʣ�Ϊ̽����ɷ֣�ij��ѧ��ȤС���ͬѧȡ������A��ĩ����ʵ�飮����ɷ������÷�ĩ������O��K��Fe����Ԫ�أ���ȡ3.96g������A�ķ�ĩ����ˮ���μ�������ϡ���ᣬ��Ӧ�����Һ�м��뺬��0.08mol��KOH����Һ��ǡ����ȫ��Ӧ�����ˣ���ϴ�Ӻ�ij���������գ��õ�����ɫ�����ĩ1.60g����������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ����10.44g����1��������A�Ļ�ѧʽΪK2FeO4������A��H2O��Ӧ�����ӷ���ʽΪ��4FeO42-+10H2O=4Fe��OH��3��+3O2��+8OH-��
��2��������A����Ϊһ�֡���ɫ��Ч��ܡ�ˮ������������FeCl3��KClO��ǿ���������·�Ӧ�Ƶã��䷴Ӧ�����ӷ���ʽΪ2Fe3++3ClO-+10OH-=2FeO42-+3Cl?+5H2O
��3��������A��������Ϊ��������ز��ϣ���MnO2-Zn������ƣ�A-ZnҲ������ɼ��Ե�أ�A�ڵ������Ϊ�������ϣ���缫��ӦʽΪFeO42-+3e��+4H2O=Fe��OH��3+5OH-���õ���ܷ�Ӧ�����ӷ���ʽΪ3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
��4��Ŀǰ��������Ի�����A���ȶ��Խ����˴�����̽������ȡ����һ���Ľ�չ�������������п����������Aˮ��Һ�ȶ��Ե���AD
A�������ơ����� B�����ᡡ C��Fe��NO3��3���� D��KOH
��5�������һ��ʵ�鷽�����о��¶ȶԻ�����Aˮ��Һ�ȶ��Ե�Ӱ�죺ȡ��������Ʒ�����Թܼ�ˮ�ܽ⣬�ֳ����ȷ������Թ��У��ֱ������ˮ����ˮ�У��۲����ɺ��ɫ�����Ŀ�����
���� ��1������ɫ��ĩΪ�������������ᾧˮ����Ϊ����أ��������ݼ����A�н�����O��K��Fe����Ԫ�صĸ����ȣ�����A�Ļ�ѧʽ��A���ȶ������ɵĺ��ɫ����Ϊ�������������嵥��ֻ�����������ݼ��غ㣬���ﻹ���������أ��ɴ�д������ʽ��ƽ���ɣ�
��2�����������ӱ������������Ϊ������أ�������ر���ԭΪ�Ȼ��أ�ͬʱ����H2O���ɴ�д������ʽ��ƽ���ɣ�
��3�����������������������Ϊ������أ�������ر���ԭΪ�Ȼ��أ�ͬʱ����H2O���ɴ�д������ʽ��ƽ���ɣ�
��4�����������Ϣ���ڳ��º���������£�������A�����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ��ȷ���������Aˮ��Һ�ȶ��ԣ�
��5���̶������������ı��¶ȣ������������Ŀ�����
��� �⣺��1��3.96g������A������$\frac{1.6g}{160g/mol}$��2=0.02mol�����أ�$\frac{10.44g}{174g/mol}$��2-0.08mol=0.04mol��
������$\frac{3.96g-0.02mol��56g/mol-0.04mol��39g/mol}{16g/mol}$=0.08mol��
�ء��������ĸ�����Ϊ��0.04mol��0.02mol��0.08mol=2��1��4����A�Ļ�ѧʽΪ��K2FeO4��
���������ˮ��Ӧ����������Fe��OH��3���������أ���Ӧ����ʽΪ4K2FeO4+10H2O=4Fe��OH��3+8KOH+3O2�������ӷ���ʽΪ4FeO42-+10H2O=4Fe��OH��3��+3O2��+8OH-��
�ʴ�Ϊ��K2FeO4��4FeO42-+10H2O=4Fe��OH��3��+3O2��+8OH-��
��2�����������ӱ������������Ϊ������أ�������ر���ԭΪ�Ȼ��أ�ͬʱ����H2O����Ӧ�����ӷ���ʽΪ2Fe3++3ClO-+10OH-=2FeO42-+3Cl?+5H2O��
�ʴ�Ϊ��2Fe3++3ClO-+10OH-=2FeO42-+3Cl?+5H2O��
��3��ԭ��صĸ�������������Ӧ�������缫��ӦʽΪ����FeO42-+3e��+4H2O=Fe��OH��3+5OH-�������缫��ӦΪ����Zn-2e-+2OH-=Zn��OH��2�����ݵ缫��Ӧ�ĵ����غ㣬�١�2+�ڡ�3�ϲ��õ���ط�ӦΪ��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
�ʴ�Ϊ��FeO42-+3e��+4H2O=Fe��OH��3+5OH-��3Zn+2FeO42-+8H2O=3Zn��OH��2+2Fe��OH��3+4OH-��
��4��A�����������ˮ��Һ�в��ȶ�����������Һ�Լ��Կ����¶ȴ��ڣ���A��ȷ��
B���������ƾ��л�ԭ�ԣ��ᱻ���������������B����
C���ڳ��º���������£�������ؿ����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ�����C����
D����������ڼ������������ɣ���D��ȷ��
�ʴ�Ϊ��AD��
��5���̶������������ı��¶ȣ������������Ŀ����������ʵ��Ϊȡ��������Ʒ�����Թܼ�ˮ�ܽ⣬�ֳ����ȷ������Թ��У��ֱ������ˮ����ˮ�У��۲����ɺ��ɫ�����Ŀ�����
�ʴ�Ϊ��ȡ��������Ʒ�����Թܼ�ˮ�ܽ⣬�ֳ����ȷ������Թ��У��ֱ������ˮ����ˮ�У��۲����ɺ��ɫ�����Ŀ�����
���� ���⿼��ѧ���Ķ���Ŀ��ȡ��Ϣ���������������������ʵ�������ȣ���Ŀ���ѣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ��������������

| A�� | ��ǿ���������������������Ľ���ͼ�� | |
| B�� | ʵʩ��ú����������ú�ĵ硱�������Դ���칤�� | |
| C�� | ��������Ч�ɳ���أ���չ���綯���� | |
| D�� | �Ӹ߹������̴ѣ�ʹ�̳��ͷ���Զ��ر� |
| A�� | �챦ʯ������ʯ����Ҫ�ɷ���Al2O3��ʯӢ����������ɸ����Ҫ�ɷ��ǹ����� | |
| B�� | ���쵰�ס���˿����������ø������ͳ��ˮ�����ɵõ������� | |
| C�� | 2014��1�£������״ν���������������Ȼ�������ͨ����������һ�ַ�ɢϵ�����ɢ��Ϊ���� | |
| D�� | ������̼�����������ᡢʳ����һ�������¾��ܵ��磬��ֻ�������ʳ�����ڵ���ʣ�������̼�Ͱ������ڷǵ���� |
| A�� | þ��2MgO �����ڣ�$\stackrel{ֱͨ����}{��}$Mg+O2�� | B�� | �ƣ�CaO+C$\stackrel{����}{��}$Ca+CO�� | ||
| C�� | �̣�3MnO2+4Al$\stackrel{����}{��}$3Mn+2Al2O3 | D�� | ����HgS$\stackrel{����}{��}$Hg+S |
| A�� | ���³�ѹ�£�22.4 L NO2�к���NA������ | |
| B�� | 1 mol�ǻ��е�����Ϊ10 NA | |
| C�� | R2+��������ΪA��������ΪN����n g R�ü�̬���������к�������Ϊ$\frac{n}{A+16}$��A-N+8��NA | |
| D�� | �ڷ�ӦKIO3+6HI�TKI+3I2+3H2O�У�ÿ����3 mol I2ת�Ƶĵ�����Ϊ6 NA |
| A�� | HClO�Ľṹʽ��H-Cl-O | B�� | Cl-�ṹʾ��ͼ��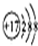 | ||
| C�� | CO2�ı���ģ�ͣ� | D�� | ������Ϊ23����ԭ�ӣ�${\;}_{23}^{11}$Na |
 ����ش��������⣺
����ش��������⣺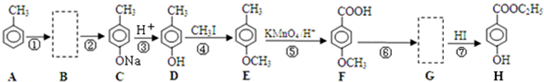
 ��
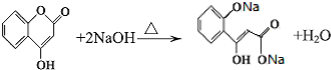
�� ����һ�������·����ۺϷ�Ӧ���ɸ߷��ӻ����д���÷�Ӧ�Ļ�ѧ����ʽ
����һ�������·����ۺϷ�Ӧ���ɸ߷��ӻ����д���÷�Ӧ�Ļ�ѧ����ʽ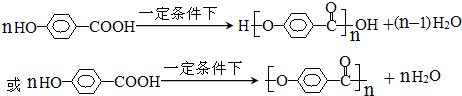 ��
��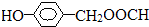 ��
��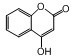 ��һ��ҽѧ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�
��һ��ҽѧ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�
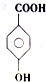
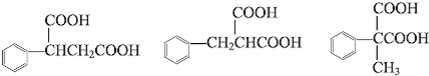 �е�����һ�֣�
�е�����һ�֣�