��Ŀ����
��һ���¶��£�10mL0.40mol/L H2O2�������ֽ⡣��ͬʱ�̲ⶨ����O2�������������Ϊ��״�������±���
������������ȷ���ǣ���Һ����仯���Բ��ƣ�
A��0~6min��ƽ����Ӧ���ʣ�v��H2O2��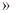
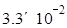 mol/(L��min)
mol/(L��min)
B��6~10min��ƽ����Ӧ���ʣ�v��H2O2����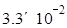 mol/(L��min)
mol/(L��min)
C����Ӧ��6minʱ��c��H2O2��=0.3mol/L
D����Ӧ��6minʱ��H2O2�ֽ���50%
| t/min | 0 | 2 | 4 | 6 | 8 | 10 |
| V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
A��0~6min��ƽ����Ӧ���ʣ�v��H2O2��
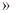
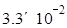 mol/(L��min)
mol/(L��min)B��6~10min��ƽ����Ӧ���ʣ�v��H2O2����
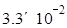 mol/(L��min)
mol/(L��min)C����Ӧ��6minʱ��c��H2O2��=0.3mol/L
D����Ӧ��6minʱ��H2O2�ֽ���50%
C
���������0~6minʱ���ڣ���c��H2O2��=0.002mol��0.01L=0.2mol/L������v��H2O2��=0.2mol/L��6min
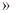
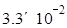 mol/(L��min)�� A��ȷ�����ŷ�Ӧ�Ľ��У�H2O2��Ũ����С����Ӧ���ʼ�����B��ȷ��6minʱ��c��H2O2��=0.002mol��0.01L=0.2mol/L�� C����6minʱ��H2O2�ֽ���Ϊ��
mol/(L��min)�� A��ȷ�����ŷ�Ӧ�Ľ��У�H2O2��Ũ����С����Ӧ���ʼ�����B��ȷ��6minʱ��c��H2O2��=0.002mol��0.01L=0.2mol/L�� C����6minʱ��H2O2�ֽ���Ϊ��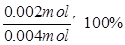 =50%�� D��ȷ��
=50%�� D��ȷ��
��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
 ?pC(g)��2D(g)������0��5 mol D����֪C��ƽ����Ӧ������0��1 mol/(L��s)��������˵������ȷ����
?pC(g)��2D(g)������0��5 mol D����֪C��ƽ����Ӧ������0��1 mol/(L��s)��������˵������ȷ���� N2(g)+3H2(g)����673K��30MPa�£�n(NH3)��n(N2)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ������������ȷ����
N2(g)+3H2(g)����673K��30MPa�£�n(NH3)��n(N2)��ʱ��仯�Ĺ�ϵ��ͼ��ʾ������������ȷ���� 
 M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���
 3Z(g) ��H��0��W��X������Ӧ���ʵ���M��X������Ӧ����
3Z(g) ��H��0��W��X������Ӧ���ʵ���M��X������Ӧ����
 2HF(g)����H>0����ƽ����ϵ��������(m��)�������ʵ���(n��)֮���ڲ�ͬ�¶�����ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����
2HF(g)����H>0����ƽ����ϵ��������(m��)�������ʵ���(n��)֮���ڲ�ͬ�¶�����ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����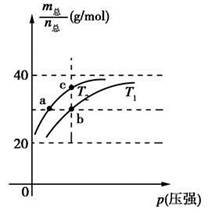
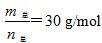 ʱ��n(HF):n[(HF)2]��2:1
ʱ��n(HF):n[(HF)2]��2:1