��Ŀ����
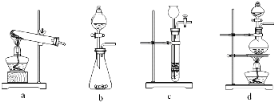
����Ŀ��ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ�飨ͼ��a��b��c��ʾֹˮ�У�
![]()

��1���ס����������������� ______�� _______��
��2���ڼ���װ��Ũ���ᣬ����װ��ͭƬ������A��C��E����ʵ�飬д��A�з�����Ӧ�Ļ�ѧ����ʽ___��������װ�����Ʒ����Һ����Ӧ���������_____��������װ��������Ը��������Һ����Ӧ����Һ��ɫ��˵�����ɵ�������___�ԣ�������װ�������������Һ����Ӧ����Һ����ǣ�˵������������____�ԡ�
��3��B��D��Eװ����������B��ʢװŨ�����ͭƬ��ͭƬ�����п����ϰ��ϣ����Ƶ�NO2�������й�ʵ�顣
��Ҫ�ڶ����ռ�NO2���壬Ӧ��ȡ�IJ�������Ϊ���ر�ֹˮ��___����ֹˮ��____��
������Dװ����֤NO2��ˮ�ķ�Ӧ�����������Ϊ���ȹر�ֹˮ��ab���ٴ�ֹˮ��c��ʹ�ձ��е�ˮ�����Թܶ��еIJ�����_____��
���Թܶ��е�NO2��ˮ��ַ�Ӧ��������Һ�����ʵ����ʵ���Ũ����____������2λ��Ч��ֵ�����尴��״�����㣩��
���𰸡���Һ©�� Բ����ƿ 2H2SO4��Ũ��+Cu![]() CuSO4+SO2��+2H2O Ʒ����Һ��ɫ ��ԭ ���� c a��b ���������Թܶ���ʹNO2�ݳ��Ӵ����ձ��е�ˮ 0.045mol/L
CuSO4+SO2��+2H2O Ʒ����Һ��ɫ ��ԭ ���� c a��b ���������Թܶ���ʹNO2�ݳ��Ӵ����ձ��е�ˮ 0.045mol/L
��������
��1�����������Ĺ����֪���ס��������������Ʒֱ��Ƿ�Һ©����Բ����ƿ��
��2��A��ͭƬ��Ũ���Ṳ�ȷ�Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ2H2SO4��Ũ��+Cu![]() CuSO4+SO2��+2H2O��������װ�����Ʒ����Һ������������ʹƷ����Һ��ɫ����Ӧ���������Ʒ����Һ��ɫ��������װ��������Ը��������Һ����Ӧ����Һ��ɫ��˵�����ɵ������л�ԭ�ԣ�������װ�������������Һ����Ӧ����Һ����ǣ�˵�����������������ԣ�
CuSO4+SO2��+2H2O��������װ�����Ʒ����Һ������������ʹƷ����Һ��ɫ����Ӧ���������Ʒ����Һ��ɫ��������װ��������Ը��������Һ����Ӧ����Һ��ɫ��˵�����ɵ������л�ԭ�ԣ�������װ�������������Һ����Ӧ����Һ����ǣ�˵�����������������ԣ�
��3����ͭ��Ũ���ᷴӦ��������ͭ������������ˮ������ʽΪCu+4HNO3��Ũ��=Cu��NO3��2+2NO2��+2H2O����Ӧ���ӷ���ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
�������������ܺ�ˮ��Ӧ�����ձ��������ѹǿ���С��С��������ѹ���ձ��е���Һ�ᵹ�����Թܶ��������ȹر�ֹˮ�� ab�ٴ�ֹˮ�� c��˫�ֽ��գ����ȣ��Թܶ�ʹ�Թ��������ݳ���NO2��ˮ�Ӵ���ˮ������ʹ�ձ��е�ˮ�����Թܶ���
�������������ΪVL�����Զ���������������ʵ���Ϊn=![]() ������������������ˮ��Ӧ�������ᣬ�������������
������������������ˮ��Ӧ�������ᣬ�������������
��������������ʵ���Ϊxmol��
4NO2+O2+2H2O=4HNO3
4mol 4mol
![]() xmol
xmol
x=![]() ��
��
c=![]() =
=![]() ��0.045mol/L��
��0.045mol/L��

����Ŀ������ͼ,����������̽�����������ʡ�ʵ��ʱ��NaOH�����ϵμ���Ũ��ˮ,��������һ������������档�±��ж�ʵ�����������Ľ�����ȷ����
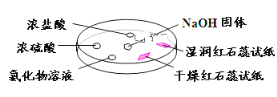
ѡ�� | ʵ������ | ���� |
A | �����ɫʯ����ֽ����ɫ,ʪ���ɫʯ����ֽ���� | NH3��һ�ֿ����Լ� |
B | Ũ���ḽ������������ | NH3��Ũ���������Ӧ |
C | �Ȼ�����Һ����� | ����Һһ����MgCl2��Һ |
D | Ũ���ḽ���������� | NH3��Ũ����ӷ�����HCl��Ӧ������NH4Cl���� |
A. A B. B C. C D. D