��Ŀ����
����Ŀ��ȫ����ÿ�걻��ʴ��ĵĸ������dz����ˣ��ڳ�ʪ�����з���������ʴ�Ǹ�����ʴ����Ҫԭ��
��1���ڳ�ʪ�����У���������������ʴʱ��������ӦʽΪ________________________��
��2����֪����ֽ�Ļ�ѧ����ʽΪ��H2C2O4![]() CO�� + CO2�� + H2O������װ���У�����������ֽ���ȡ�������________________��
CO�� + CO2�� + H2O������װ���У�����������ֽ���ȡ�������________________��
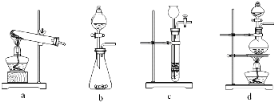
��3��ijʵ��С�����ò���ֽ������CO�����ⷴӦ���ⶨ������Ʒ����ɣ��ٶ�������ֻ��Fe2O3�� nH2O��Fe���ֳɷݣ���ʵ��װ������ͼ��ʾ��

��Ϊ�õ����������CO���壬ϴ��ƿA��B��ʢ�ŵ�Һ̬�Լ����ο�����_______��________����𰸱�ţ���
a. Ũ���� b.�����ʯ��ˮ c. ����������Һ d. ��ˮ�Ȼ���
���ڵ�ȼC���ƾ���֮ǰӦ���еIJ����ǣ���a�����װ�������ԣ���b��____________��
��Eװ�õ�������___________________________________________________________ ��
��ȷ������Ʒ10.00g����Ӳ�ʲ������У���ַ�Ӧ����ȴ������������ÿ������ȫ��Ӧ����Ӳ�ʲ�������ʣ���������Ϊ8.32 g��D��Ũ��������0.72 g����n =_____________��
���ڱ�ʵ���У����������ʹ�ⶨ���nƫ�����__________����𰸱�ţ���
a��ȱ��װ��B �� b��ȱ��װ��E �� c����Ӧ��Ĺ�����������Fe2O3��nH2O
���𰸡�O2+ 4e��+2H2O= 4OH��dcaͨ��������һ��ʱ�䣬�ų���ϵ�еĿ�����ֹ�����е�H2O������ϵ��Ӱ��ʵ����2a
��������
��1����������������ʴ���������������õ����������������ӣ��缫��ӦΪ��O2+ 4e��+2H2O= 4OH����
��2���÷�Ӧ�ķ�Ӧ����Һ�壬��Ӧ�����Ǽ��ȣ�Ӧѡ���Һ��Ӧ����Ҫ���ȵ�װ�ã�����ѡd��
��3����Ϊ�õ����������CO����Ӧ�ȳ�ȥ��������еĶ�����̼���������������ռ��ɣ��������ͨ������������Һʱ�����ˮ������Ϊ��ȥˮ������ѡ��Ũ����ϴ�����ʴ�Ϊ��c��a��
��Ϊ��ֹ�����Է�Ӧ��Ӱ�죬Ӧ�ȳ�ȥ��װ���еĿ���������Ҫͨ��������һ��ʱ�䣬
�ʴ�Ϊ��ͨ��������һ��ʱ�䣬�ų���ϵ�еĿ�������ֹ����ʱCO������ը��
��Eװ�÷�ֹ�����еĶ�����̼��ˮ��������װ��Ӱ��ʵ������һ����̼�Ĵ���������������ȼ��ȥ���ʴ�Ϊ����ֹ�����е�CO2��H2O������ϵ��Ӱ��ʵ������
��������֪�������������ٵ���Ϊˮ����������Ũ�������յ���Ϊˮ����Ӧ����ʽΪ��
Fe2O3��nH2O+3CO=Fe+3CO2+nH2O �������ٵ���Ϊ
18ng ��18n+48��g
0.72g 10.00g-8.32g
����0.72g����18n+48��g=18ng���� 10.00g-8.32g�������n=2��
��a��ȱ��ϴ��ƿB�ᵼ��װ��E���ص�����ƫ�����Բⶨ���nƫ��a��ȷ��b����ʯ�����յ��Ƿ�Ӧ��Ķ�����̼����������ֵ�أ�������Ӱ�죬��b����c����Ӧ���������������Fe2O3��nH2O��������ٵ�����ƫС����������nֵƫС����c���ʴ�Ϊ��a��

 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�����Ŀ��NiΪ�ڢ� ��Ԫ�أ������ҵ�����ж����漰��
��֪���������壨NiC2O4��2H2O��������ˮ����ҵ�ϴӷ����������ɷ���ҪΪNi������һ������Al2O3��Fe��SiO2��CaO�ȣ��Ʊ������������������ͼ��ʾ��

��֪������ؽ������������������������pH���±���
�������� | Fe3+ | Fe2+ | Al3+ | Ni2+ |
��ʼ������pH | 1.1 | 5.8 | 3.0 | 6.8 |
��ȫ������pH | 3.2 | 8.8 | 5.0 | 9.5 |
��Ksp(CaF2)=1.46��10-10 �۵�ij����Ũ��С��1.0��10-5 mol/Lʱ����Ϊ��ȫ����
��1����д��һ�������������ʵĴ�ʩ________________��
��2���Լ�a��һ����ɫ��������д����������ʱ��Ӧ�����ӷ���ʽ__________________________��
��3��pH�ĵ��ط�ΧΪ __________________��������ijɷ�Ϊ_____________________________��
��4��д����������ʱ��Ӧ�����ӷ���ʽ___________________________________________��֤��Ni2���Ѿ�������ȫ��ʵ�鲽�輰������______________________________________����Ca2+������ȫʱ����Һ��c(F��)>_____________ mol/L��д������ʽ���ɣ���
��5������a��������____________________________________________��




