��Ŀ����
11��ͨ��ѪҺ�еĸ����ӵļ���ܹ������ж϶��ּ�����ij�о�С��Ϊ�ⶨѪҺ��Ʒ��Ca2+�ĺ�����100mLѪҺ�к�Ca2+����������ʵ�鲽�����£�
��ȷ��ȡ5.00mLѪҺ��Ʒ�����������Ƴ�50.00mL��Һ��
��ȷ��ȡ��Һ10.00mL�����������NH4��2C2O4��Һ��ʹCa2+��ȫת����CaC2O4������
�۹��˲�ϴ������CaC2O4�������ù���ϡ�����ܽ⣬����H2C2O4��CaSO4ϡ��Һ��
�ܼ���12.00mL 0.0010mol•L-1��KMnO4��Һ��ʹH2C2O4��ȫ�����������ӷ���ʽΪ��
2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O��
����0.0020mol•L-1 ��NH4��2Fe��SO4��2��Һ�ζ�������KMnO4��Һ�����ģ�NH4��2Fe��SO4��2��Һ20.00mL��
��1����֪������CaC2O4��Ksp=2.0��10-9����ʹ�������
c��Ca2+����1.0��10-5 mol•L-1��Ӧ������Һ��c��C2O42-����2.0��10-4mol•L-1��
��2�����������Mn2+���ɣ�������Ӧ�����ӷ���ʽΪMnO4-+8H++5Fe2+=Mn2++5Fe3++4H2O��
��3��������ݵζ�����ʹ��ǰδ�ñ���NH4��2Fe��SO4��2��Һϴ�ӣ����ѪҺ��Ca2+�ĺ�����ƫ�ͣ��ƫ�ߡ�����ƫ�͡����䡱����
��4������Ѫ����Ca2+�ĺ���0.04g/mL��д��������̣���
���� ��1�������ܶȻ�����Ksp=c��C2O42-��•c��Ca2+�������㣻
��2����NH4��2Fe��SO4��2��Һ�е�Fe2+���н�ǿ�Ļ�ԭ�ԣ��ܱ����Ը��������Һ����ΪFe3+��
��3��������ݵζ�����ʹ��ǰδ�ñ���NH4��2Fe��SO4��2��Һϴ�ӣ�����ζ��ܱ�������ˮ��ע��ı���NH4��2Fe��SO4��2��Һ�ᱻϡ�ͣ����ĸ���ı���NH4��2Fe��SO4��2��Һ�����ʣ��KMnO4��Һƫ�࣬KMnO4��Һ����H2C2O4ƫ�٣����Բ��ѪҺ��Ca2+�ĺ�����ƫ�ͣ�
��4�����ݣ�2���з���ʽ���������KMnO4�����ʵ������Ӷ��õ���H2C2O4��Ӧ��KMnO4�����ʵ�����Ȼ�����2MnO4-+5H2C2O4+6H+�T10CO2��+2Mn2++8H2O���n��CaC2O4��=n��H2C2O4��=n��Ca2+��������m=nM����������
��� �⣺��1����Ksp=c��C2O42-��•c��Ca2+����֪����ʹc��Ca2+����1.0��10-5mol•L-1��Ӧ������Һ��c��C2O42-����$\frac{{K}_{sp}}{c��C{a}^{2+}��}$=$\frac{2.0��1{0}^{-9}}{1.0��1{0}^{-5}}$mol/L=2.0��10-4 mol/L��
�ʴ�Ϊ��2.0��10-4��
��2����NH4��2Fe��SO4��2��Һ�е�Fe2+���н�ǿ�Ļ�ԭ�ԣ��ܱ����Ը��������Һ����ΪFe3+�����ӷ���ʽΪ��MnO4-+8H++5Fe2+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��MnO4-+8H++5Fe2+=Mn2++5Fe3++4H2O��
��3��������ݵζ�����ʹ��ǰδ�ñ���NH4��2Fe��SO4��2��Һϴ�ӣ�����ζ��ܱ�������ˮ��ע��ı���NH4��2Fe��SO4��2��Һ�ᱻϡ�ͣ����ĸ���ı���NH4��2Fe��SO4��2��Һ�����ʣ��KMnO4��Һƫ�࣬KMnO4��Һ����H2C2O4ƫ�٣����Բ��ѪҺ��Ca2+�ĺ�����ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�
��4��KMnO4�������ʵ���Ϊ��0.0010 mol•L-1��12��10-3L=1.2��10-5 mol��
��NH4��2Fe��SO4��2��Һ�ζ����ĵĹ�����KMnO4�����ʵ���Ϊ��0.0020 mol•L-1��20.00��10-3L��$\frac{1}{5}$=8.0��10-6 mol��
��H2C2O4��Ӧ��KMnO4����Ϊ��1.2��10-5 mol-8.0��10-6 mol=4.0��10-6 mol��
n��H2C2O4��=4.0��10-6 mol��$\frac{5}{2}$=1.0��10-5 mol��n��CaC2O4��=1.0��10-5 mol��
����Ѫ����Ca2+�ĺ���Ϊ��1.0��10-5 mol��40 g•mol-1��$\frac{50ml}{10ml}$��$\frac{100ml}{5ml}$=0.04g/L��
�ʴ�Ϊ��0.04g/mL��
���� ���⿼�����ܶȻ������ļ��㡢���ӷ�Ӧ����ʽ����д�Լ����㣬�ۺ��Խ�ǿ����ȷ��д���ӷ�Ӧ����ʽ�Ǽ���Ĺؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | pH=1����Һ�У�Fe2+��NO3-��Na+��SO42- | |
| B�� | ˮ�������c��H+��=10-12mol/L����Һ�У�Ca2+��K+��Cl-��HCO3- | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012��ˮ��Һ�У�NH4+��Al3+��NO3-��Cl- | |
| D�� | c��Fe3+��=0.1mol/L����Һ�У�K+��ClO-��SO42-��SCN- |
������0.50mol•L-1NaOH��Һ
��1����ʵ����Լ��Ҫ240ml0.50mol•L-1NaOH��Һ����Ӧ����Ͳ��ȡ2.5mol•L-1NaOH��Һ�����Ϊ50.0mL��
��2������0.50mol•L-1NaOH��Һʱ����Ҫʹ�õIJ�����������Ͳ���ձ����������⣬����250mL����ƿ����ͷ�ιܣ�
�ⶨ�к���
ȡ60mL NaOH��Һ��40mL������Һ����ʵ�飬ʵ�����������
| ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | ||
| H2SO4 | NaOH | ƽ��ֵ ������С�����һλ�� | ||
| 1 | 26.3 | 26.0 | 26.1 | 30.1 |
| 2 | 27.0 | 27.3 | 27.2 | 33.3 |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 |
| 4 | 26.5 | 26.2 | 26.3 | 30.4 |
��4������ʵ����ֵ�����57.3kJ•mol-1��ƫ�������ƫ���ԭ�����ad������ĸ����
a��ʵ��װ�ñ��¡�����Ч����
b����ȡ40mL0.50mol•L-1����ʱ���Ӷ���
c�����ᵹ��С�ձ�ʱ�����������ὦ��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��5���ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ��1L 0.50mol/L��ϡ����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�Ϊ��H1����H2����H3�����H1����H2����H3�ɴ�С�Ĺ�ϵΪ��H3����H1����H2��
| A�� | SiO2 CsCl CBr4 CF4 | B�� | SiO2 CsCl CF4 CBr4 | ||
| C�� | CsCl SiO2 CBr4 CF4 | D�� | CF4 CBr4 CsCl SiO2 |
| A�� | ��˾ƥ�� | B�� | �������� | C�� | ��� | D�� | ��ù�� |
| A�� | Ϊ�˼�����Ӧ���ʿ��ñ���ʳ��ˮ����ˮ���з�Ӧ | |
| B�� | �÷�ӦΪ���ȷ�Ӧ | |
| C�� | ������Ȳ�ķ�ӦΪCaC2+H2O��CaO+CH��CH�� | |
| D�� | Ϊ�˳�ȥ��Ȳ�����е����ʿ�����CuSO4��Һϴ�� |
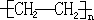 ����Ӧ�����ǼӾ۷�Ӧ��
����Ӧ�����ǼӾ۷�Ӧ�� _��
_��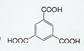 ��
��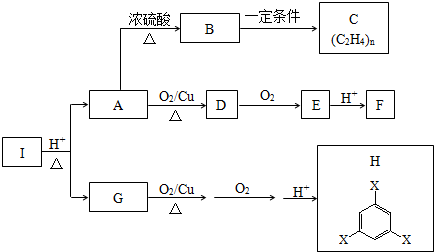
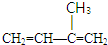 ��
��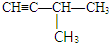 ��
��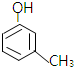 ��
��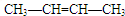 ��
��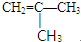 ��
�� ��
��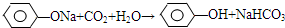
 �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ�����AC
���õ�ԭʼԭ�Ͽ�����AC