��Ŀ����
[��ѧ-���ʽṹ������]
��Ԫ��B�ڻ�ѧ���к���Ҫ�ĵ�λ����Ļ�������ũҵ��ҽԺ�������ȷ�����;�ܹ㣮��ش��������⣺
��1��д����BԪ��ͬ�����GaԪ�صĻ�̬ԭ�Ӻ�����ӷֲ�ʽ______����ԭ�ӽṹ�ĽǶȷ�����B��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ______��
��2������������������˹������ڸ��¸�ѹ�����ºϳɣ����ڳ�Ӳ���ϣ�ͬ��ԭ�Ӿ���ĵ�����BN���Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ����______��
��3����BF3����������ԭ�ӵ��ӻ����������______��SiF4���Ŀռ乹����______��
��4����ѧ�ҷ�����þ��39Kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������У���ͼ��ʾ�Ǹþ��������ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϣ�����ͼʾȷ����þ�Ļ�ѧʽΪ______��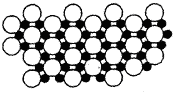
��Ԫ��B�ڻ�ѧ���к���Ҫ�ĵ�λ����Ļ�������ũҵ��ҽԺ�������ȷ�����;�ܹ㣮��ش��������⣺
��1��д����BԪ��ͬ�����GaԪ�صĻ�̬ԭ�Ӻ�����ӷֲ�ʽ______����ԭ�ӽṹ�ĽǶȷ�����B��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ______��
��2������������������˹������ڸ��¸�ѹ�����ºϳɣ����ڳ�Ӳ���ϣ�ͬ��ԭ�Ӿ���ĵ�����BN���Ⱦ������и���Ӳ�Ⱥ������Ե�ԭ����______��
��3����BF3����������ԭ�ӵ��ӻ����������______��SiF4���Ŀռ乹����______��
��4����ѧ�ҷ�����þ��39Kʱ�ʳ����ԣ�����þ���������ģ���У�þԭ�Ӻ���ԭ���Ƿֲ��Ų��ģ�һ��þһ����������У���ͼ��ʾ�Ǹþ��������ȡ���IJ���ԭ����Z�᷽���ͶӰ��������þԭ��ͶӰ����������ԭ��ͶӰ��ͼ�е���ԭ�Ӻ�þԭ��ͶӰ��ͬһƽ���ϣ�����ͼʾȷ����þ�Ļ�ѧʽΪ______��

��1��Ga��31��Ԫ�أ��������31�����ӣ����ݹ���ԭ��֪���̬ԭ�Ӻ�����ӷֲ�ʽΪ��[Ar]3d104s24p 1��ͬһ���ڣ�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�ش���������Ԫ�أ�����B��N��OԪ�صĵ�һ�������ɴ�С��˳��ΪN��O��B���ʴ�Ϊ��[Ar]3d104s24p 1��N��O��B��
��2��ԭ�Ӿ����Ӳ����ԭ�Ӱ뾶�������ɷ��ȣ�Nԭ�Ӻ�Bԭ�ӵİ뾶�ȹ�ԭ��С��B-N������Si-Si�̣����Ե�����BN���Ⱦ������и���Ӳ�Ⱥ������ԣ��ʴ�Ϊ��Nԭ�Ӻ�Bԭ�ӵİ뾶�ȹ�ԭ��С��B-N������Si-Si�̣�
��3����BF3������Bԭ�ӵļ۲���Ӷ�=3����û�йµ��Ӷԣ���������ԭ�ӵ��ӻ����������sp2�ӻ���SiF4�й�ԭ�Ӻ���4�����ۼ����Ҳ����µ��Ӷԣ��������Ŀռ乹�������������ͣ��ʴ�Ϊ��sp2���������壻
��4������ͶӰ��֪��1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�������һ��Mgԭ�ӵ�Bԭ��Ϊ
��1��Mgԭ��Ϊ6��Bԭ�ӹ��ã�������һ��Bԭ�ӵ�Mgԭ��Ϊ
���ɴ˿�֪Mgþ��ԭ�Ӹ�����=
��
=1��2������þ�Ļ�ѧʽΪMgB2���ʴ�Ϊ��MgB2��
��2��ԭ�Ӿ����Ӳ����ԭ�Ӱ뾶�������ɷ��ȣ�Nԭ�Ӻ�Bԭ�ӵİ뾶�ȹ�ԭ��С��B-N������Si-Si�̣����Ե�����BN���Ⱦ������и���Ӳ�Ⱥ������ԣ��ʴ�Ϊ��Nԭ�Ӻ�Bԭ�ӵİ뾶�ȹ�ԭ��С��B-N������Si-Si�̣�
��3����BF3������Bԭ�ӵļ۲���Ӷ�=3����û�йµ��Ӷԣ���������ԭ�ӵ��ӻ����������sp2�ӻ���SiF4�й�ԭ�Ӻ���4�����ۼ����Ҳ����µ��Ӷԣ��������Ŀռ乹�������������ͣ��ʴ�Ϊ��sp2���������壻
��4������ͶӰ��֪��1��Bԭ��Ϊ3��Mgԭ�ӹ��ã�������һ��Mgԭ�ӵ�Bԭ��Ϊ
| 1 |
| 3 |
| 1 |
| 6 |
| 1 |
| 6 |
| 1 |
| 3 |

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ

 ��ƽ��ṹ������
��ƽ��ṹ������ ��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�
��2011?����ģ�⣩����ѧ--���ʽṹ�����ʡ�