��Ŀ����
8��Na2S2O3•5H2O����Ϊ��Ч���ȼ�����ҵ����������FeS2��Ϊԭ���Ʊ������ʵ��������£�
��֪��
������A����ʹƷ����Һ��ɫ�������⣨H2S������ܻ�õ�����
��pHԼΪ11�������£����������������ο��Թ���������������Σ�
�ش��������⣺
��1������¯�н�������������ÿ�������ʹ֮�ﵽ�����ڡ�״̬����Ŀ����ʹ�����������ֽӴ����ӿ췴Ӧ���ʣ�
��2���������е�ԭ��B����ѡ��B������ĸ��ţ���
A��NaCl��Һ B��Na2CO3��Һ C��Na2SO4��Һ
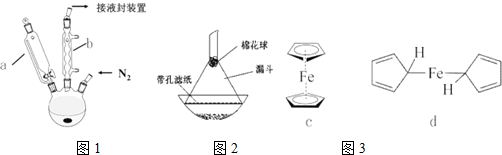
��3��ijС��ͬѧ����ͼװ��ģ���Ʊ�Na2S2O3 �Ĺ��̣�����װ������ȥ����
��A��ʹ��70%���������98%��Ũ���ᷴӦ���ʿ죬��ԭ���Ǹ÷�Ӧ��ʵ����H+��SO32-��Ӧ��70%�������к�ˮ�϶࣬c��H+����c��SO32-�����ϴ�����SO2���ʸ��죮װ��B�������Ƿ�ֹ������
��C���Ʊ�Na2S2O3������������Ӧ�У�Na2S+H2O+SO2�TNa2SO3+H2S��2H2S+SO2�T3S��+2H2O �� 2H2S+H2SO3�T3S��+3H2O��Na2SO3+S $\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��
��4������ʦ����˴��������õ�����Ĺ��գ���������������ù�����ϡ�����ȡ���õ���������������壬�÷�Ӧ�Ļ�ѧ����ʽΪFeS2+2HCl�TFeCl2+H2S��+S��
���� �������ڷ���¯��ȼ�������������������������AΪ����������ҺD����Ũ������ȴ�ᾧ�õ���������ƾ��壬��DΪNa2S2O3�����ʵ��������������ο��Թ���������������Σ�����ҺCΪNa2SO3����ԭ��BΪ����Ϊ̼������Һ������������Һ��
��1��ʹ�����������ֽӴ����ӿ췴Ӧ���ʣ�
��2��Bϡ�Ͷ�������ת��Ϊ�������ƣ�
��3��AΪ�Ʊ�����������������C����������Һ��Ӧ�õ����⣬�������������Ӧ�õ�S���ʣ�S�������������Ʒ�Ӧ�õ���������ƣ�C�з�Ӧ����װ����ѹǿ��С��Bװ�������Ƿ�ֹ������
��4������Ŀ��Ϣ��֪��FeS2��HCl��Ӧ����H2S��S��ͬʱ������FeCl2��
��� �⣺�������ڷ���¯��ȼ�������������������������AΪ����������ҺD����Ũ������ȴ�ᾧ�õ���������ƾ��壬��DΪNa2S2O3�����ʵ��������������ο��Թ���������������Σ�����ҺCΪNa2SO3����ԭ��BΪ����Ϊ̼������Һ������������Һ��
��1������¯�н�������������ÿ�������ʹ֮�ﵽ�����ڡ�״̬����Ŀ���ǣ�ʹ�����������ֽӴ����ӿ췴Ӧ���ʣ�
�ʴ�Ϊ��ʹ�����������ֽӴ����ӿ췴Ӧ���ʣ�
��2��B���ն�������ת��Ϊ�������ƣ�̼���ƿ������������Ӧ���������������������NaCl�������Ʋ����������Ӧ����ѡ��B��
��3��AΪ�Ʊ�����������������C����������Һ��Ӧ�õ����⣬�������������Ӧ�õ�S���ʣ�S�������������Ʒ�Ӧ�õ���������ƣ�C�з�Ӧ����װ����ѹǿ��С��Bװ�������Ƿ�ֹ������
�ٸ÷�Ӧ��ʵ����H+��SO32-��Ӧ��70%�������к�ˮ�϶࣬c��H+����c��SO32-�����ϴ�����SO2���ʸ��죻Bװ�������Ƿ�ֹ������
�ʴ�Ϊ���÷�Ӧ��ʵ����H+��SO32-��Ӧ��70%�������к�ˮ�϶࣬c��H+����c��SO32-�����ϴ�����SO2���ʸ��죻B��ֹ������
��C���Ʊ�Na2S2O3������������Ӧ�У�Na2S+H2O+SO2�TNa2SO3+H2S��2H2S+SO2�T3S��+2H2O �� 2H2S+H2SO3�T3S��+3H2O ��Na2SO3+S $\frac{\underline{\;\;��\;\;}}{\;}$Na2S2O3��
�ʴ�Ϊ��2H2S+SO2�T3S��+2H2O �� 2H2S+H2SO3�T3S��+3H2O��
��4������Ŀ��Ϣ��֪��FeS2��HCl��Ӧ����H2S��S��ͬʱ������FeCl2����Ӧ����ʽΪ��FeS2+2HCl�TFeCl2+H2S��+S��
�ʴ�Ϊ��FeS2+2HCl�TFeCl2+H2S��+S��
���� ���⿼���Ʊ�������ƣ����ؿ���ѧ���Բ������衢ԭ���ķ������ۣ��Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�| A�� | FeS | B�� | CuS | C�� | CuCl2 | D�� | FeCl3 |
| A�� | �ð�ˮ���չ�����SO2��2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| B�� | Ca��HCO3��2 ��Һ�м�����������ʯ��ˮ��HCO3-+Ca2++OH-=CaCO3��+H2O | |
| C�� | FeI2��Һ��ͨ������Cl2��2Fe2++Cl2=2Fe3++2Cl- | |
| D�� | NaClO��Һ��FeCl2��Һ��ϣ�2ClO-+Fe2++2H2O=Fe��OH��2��+2HClO |
| A�� | 3�� | B�� | 6�� | C�� | 9�� | D�� | 10�� |
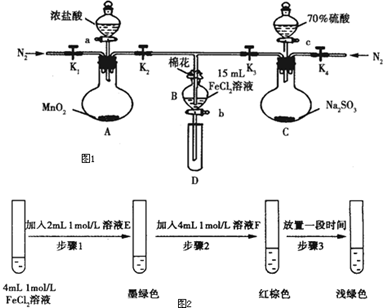
ʵ����̣�
���ɼ�K1��K4��ͨ��һ��ʱ��N2���ٽ�T�͵��ܲ���B�У�����ͨ��N2��Ȼ��ر�K1��K2��K4��
����a���μ�һ������Ũ���ᣬ��A���ȣ�
��B����Һ���ʱ��ֹͣ���ȣ��н����ɼ�K2��
��������b��ʹԼ2ml����Һ����D�Թ��У��������е����ӣ�
�������ɼ�K2������c������70%�����ᣬһ��ʱ���н����ɼ�K2��
���������Թ�D���ظ����̢�������B��Һ�е����ӣ�
��1�����̢��Ŀ�����ų�װ���еĿ�������ֹ����ʵ�飮
��2�����н������ҺΪNaOH��Һ��
��3��A�з�����Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O��
��4����70%��������ȡSO2����Ӧ���ʱ���98%������죬ԭ����70%������������Ũ�ȴ���98%�����ᣬ��˷�Ӧ���ʸ��죮
��5���ס��ҡ�����λͬѧ�ֱ����������ʵ�飬�������±���ʾ�����ǵļ����һ���ܹ�֤��������Cl2��Fe3+��SO2�����ҡ�������ס������ҡ���������
| ���̢���B��Һ�к��е����� | ���̢���B��Һ�к��е����� | |
| �� | ��Fe3+����Fe2+ | ��SO42- |
| �� | ����Fe3+������Fe2+ | ��SO42- |
| �� | ��Fe3+����Fe2+ | ��Fe2+ |
�������ϣ�Fe2+��aq��+2SO32-��aq��?FeSO3��s����ī��ɫ��
������裺FeCl2��SO2�ķ�Ӧ�������м����FeSO3����Һ�ĺ���ɫ��FeSO3��ī��ɫ����FeCl3����ɫ���Ļ��ɫ��
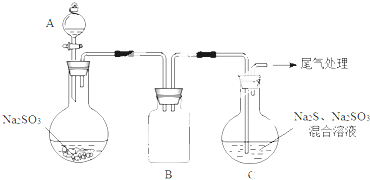
ijͬѧ�����ͼ2ʵ�飬֤ʵ�ü��������
����ҺE��F�ֱ�ΪNa2SO3��FeCl3��
���������ӷ���ʽ���Ͳ���3����Һ�����ԣ��ɺ���ɫ��dz��ɫ��ԭ��Fe3+����SO32-��c��SO32-����С��ƽ��Fe2+��aq��+SO32-��aq��?FeSO3��s����ī��ɫ�������ƶ�����Һ��ɫ�ɺ���ɫ��Ϊdz��ɫ��
| A�� | 2-���� | B�� | 2��2-����-1-���� | ||
| C�� | ������ | D�� | 2-��-1-���� |
| A�� | Ԫ�����ڱ���18�У������ҵĵ�ʮ����Ϊ±��Ԫ�� | |
| B�� | ͬһ����Ԫ�ص�ԭ�ӣ��뾶ԽСԽ����ʧȥ���� | |
| C�� | ����Ԫ��ȫ���ǽ���Ԫ�� | |
| D�� | �ڹ���Ԫ���У����ǿ���Ѱ�ҵ����������Ĵ������¡���ʴ�ĺϽ���� |