��Ŀ����
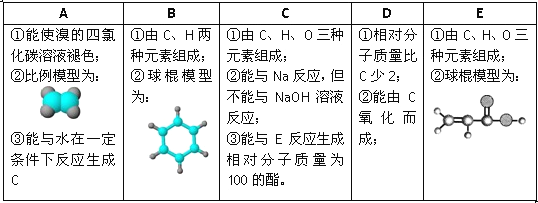
����Ŀ����Q����W�ĺϳ�·�����£�
��֪��
��
��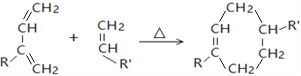
(R��R����������ԭ�ӡ������������)
��1����ϵͳ�������У�A��������_________���ϳ�A�ķ�Ӧ������__________��
��2��B�����������ŵ�������____________��
��3���Լ�b�Ľṹʽ��____________��
��4������˵���У���ȷ��____________��
�� �Լ�aΪ�״�
�� C��D��D��E��Ӧ����ǰ��
�� �γ�![]() �ĵ����к�G
�ĵ����к�G
�� ��Q���ױ�Br2��ʴ
��5��C��D�Ļ�ѧ����ʽ��___________��
��6���Լ�b�뱽���γɸ߷��ӻ�����Ļ�ѧ����ʽ��_____________��
��7��F�Ľṹ��ʽ��_____________��
��8����CH2=CH-CH=CH2��HOCH2CH=CHCHOΪԭ���Ʊ�![]() ��
��
��ϳ�·��ͼ�ǣ�____________
���𰸡� 1,2-����-2-������ �ӳ� �ǻ� ![]()
![]() ��
�� ![]() +
+ ![]() ��
��![]() + ��n-1��H2O
+ ��n-1��H2O ![]()
![]()
��������1��3������ϩ����ӳ�����A����A�Ľṹ��ʽΪ(CH3)2CBrCH2Br��Aˮ������B��B�Ľṹ��ʽΪ(CH3)2COHCH2OH��B����������C��C�Ľṹ��ʽΪ(CH3)2COHCHO��C��Ũ����������·�����ȥ��Ӧ����D����D�Ľṹ��ʽΪCH2��C(CH3)CHO��D����������Ӧ���ữ������E��E�Ľṹ��ʽΪCH2��C(CH3)COOH������W�Ľṹ��ʽ���ж��Լ�a���Ҵ�����E����������Ӧ����F��F�Ľṹ��ʽΪCH2��C(CH3)COOCH2CH3��������֪��Ϣ�ڿ�֪F��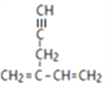 ����˫ϩ�ϳɷ�Ӧ����W��G�Ӿ�����Q�������Q�Ľṹ��ʽ��֪G�Ľṹ��ʽΪCH2��C(CH3)CH��CH2��1��3������ϩ��b������֪��Ϣ�ٵķ�Ӧ����G����b�Ǽ�ȩ��
����˫ϩ�ϳɷ�Ӧ����W��G�Ӿ�����Q�������Q�Ľṹ��ʽ��֪G�Ľṹ��ʽΪCH2��C(CH3)CH��CH2��1��3������ϩ��b������֪��Ϣ�ٵķ�Ӧ����G����b�Ǽ�ȩ��
��1����ϵͳ�������У�A��������1��2-����-2-�����顣�������Ϸ�����֪�ϳ�A�ķ�Ӧ�����Ǽӳɷ�Ӧ����2������B�Ľṹ��ʽ(CH3)2COHCH2OH��֪B�����������ŵ��������ǻ�����3���Լ�b�Ľṹʽ��HCHO����4�����Լ�aΪ�Ҵ�������C��D��D��E��Ӧ����ǰ���������γ�![]() �ĵ�������ϩ�������ϩ�������к�G����ȷ������Q������̼̼˫�����ױ�Br2��ʴ��d����ѡc����5��C��D�Ļ�ѧ����ʽ��
�ĵ�������ϩ�������ϩ�������к�G����ȷ������Q������̼̼˫�����ױ�Br2��ʴ��d����ѡc����5��C��D�Ļ�ѧ����ʽ��![]() ����6���Լ�b�뱽���γɸ߷��ӻ�����Ļ�ѧ����ʽ��n
����6���Լ�b�뱽���γɸ߷��ӻ�����Ļ�ѧ����ʽ��n![]() +n
+n![]() ��
��![]() +��n-1��H2O����7��F�Ľṹ��ʽ��CH2��C(CH3)COOCH2CH3����8�����������Ϣ˫ϩ�ϳɿ�֪��CH2=CH-CH=CH2��HOCH2CH=CHCHOΪԭ���Ʊ�
+��n-1��H2O����7��F�Ľṹ��ʽ��CH2��C(CH3)COOCH2CH3����8�����������Ϣ˫ϩ�ϳɿ�֪��CH2=CH-CH=CH2��HOCH2CH=CHCHOΪԭ���Ʊ�![]() �ĺϳ�·��ͼ�ǣ�
�ĺϳ�·��ͼ�ǣ�![]() ��
��

����Ŀ����Na2SO3��Һ�Ͳ�ͬ��������������Һ��Ϊʵ�����̽���ε����ʺ�����Һ�䷴Ӧ�Ķ����ԡ�
ʵ�� | �Լ� | ���� | |
�ι� | �Թ� | ||
2 mL | 0.2 mol��L1 Na2SO3��Һ | ����Ag2SO4��Һ | ��.������ɫ���� |
0.2 mol��L1 CuSO4 | ��.��Һ���̣������μӲ����ػ�ɫ���� | ||
0.1 mol��L1 Al2��SO4��3��Һ | ��.��ʼ�����Ա仯�������μӲ�����ɫ���� | ||
��1�������飬������еİ�ɫ������Ag2SO3�������ӷ���ʽ���������____________��
��2�������飬�������ػ�ɫ�����в���SO42������Cu+��Cu2+��SO32��
��֪��Cu+![]() Cu +Cu2+��Cu2+
Cu +Cu2+��Cu2+![]() CuI������ɫ��+I2��
CuI������ɫ��+I2��
����ϡ����֤ʵ�����к���Cu+��ʵ��������_____________��
��ͨ������ʵ��֤ʵ�������к���Cu2+��SO32��
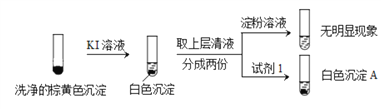
a.��ɫ����A��BaSO4���Լ�1��____________��
b.֤ʵ�����к���Cu+��SO32��������_______________��
��3����֪��Al2��SO3��3��ˮ��Һ�в����ڡ������飬�����İ�ɫ��������SO42���ð�ɫ������������ǿ�ᣬ��������ǿ�����ʹ����KMnO4��Һ��ɫ��
���Ʋ�����к������������____________��
�ڶ��ڳ�������������Ĵ�����ʽ������ּ��裺i. Al��OH��3��������ii.���������ļ�ʽ���С��Լ���ii����˶Ա�ʵ�飬֤ʵ�˼���ii������
���Ա�ʵ�鷽������������
����һ��
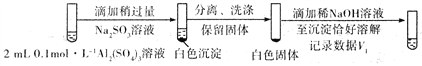
�������

��V1___________V2����>��<��=����
��4������ʵ�飬�������ε�������___________������Һ�䷴Ӧ�Ķ�������__________�йء�