��Ŀ����
����Ŀ���±���A��B��C��D��E�����л�����й���Ϣ��
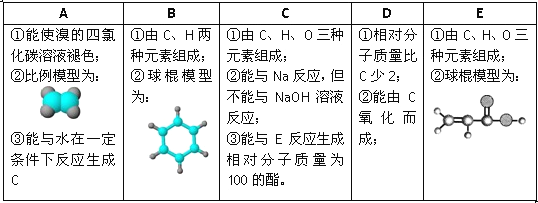
���ݱ�����Ϣ�ش��������⣺
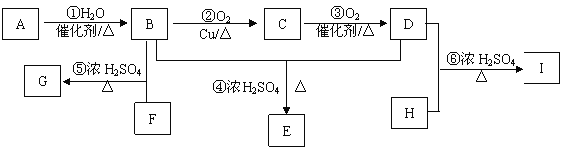
(1)A��E�У�����������_______(����ĸ)��д��A����ˮ��Ӧ�Ļ�ѧ����ʽ_____________��
(2)A�����������ӳɷ�Ӧ�����ɷ���F����F�ڷ�����ɺͽṹ�����Ƶ��л�����һ����(�׳ơ�ͬϵ�)�����Ǿ�����ͨʽ_______����n=________ʱ�������л��↑ʼ����ͬ���칹�塣
(3)B���������_____________(�����)��
����ɫ��ζҺ�� ���ж� �۲�����ˮ ���ܶȱ�ˮ��
���κ������²���������Ӧ��ʹ���Ը��������Һ����ˮ����ɫ
(4)д����Ũ���������£�B��Ũ���ᷴӦ�Ļ�ѧ����ʽ��_________________��
(5)C��E��Ӧ��������Է�������Ϊ100�������÷�Ӧ����Ϊ_____________���仯ѧ����ʽΪ��______________________��
���𰸡� A B CH2=CH2 + Br2��CH2BrCH2Br CnH2n+2 4 �ڢ� ![]() ������Ӧ(��ȡ����Ӧ) CH2��CH��COOH �� C2H5OH
������Ӧ(��ȡ����Ӧ) CH2��CH��COOH �� C2H5OH ![]() CH2��CH��COOC2H5 �� H2O(2��)
CH2��CH��COOC2H5 �� H2O(2��)
��������A��ʹ���CCl4��Һ��ɫ˵������̼̼˫������������ϱ���ģ��֪��AΪ����ϩ��A����ˮ��һ�������·�Ӧ����C����CΪ�Ҵ�������B��C��H����Ԫ����ɼ������ģ�Ϳ�֪��BΪ����D����Է���������C��2������C�������ɣ�����DΪ��ȩ����E�����Ԫ�ؼ����ģ�Ϳ�֪��EΪCH2=CH-COOH��
��1��������������5���л�����ֻ����ϩ�ͱ�����������A��E�У�������������A��B����ϩ����ˮ�����ӳɷ�Ӧ����ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��
��2��AΪ��ϩ�����������ӳɷ�Ӧ������F��FΪ���飬��F�ڷ�����ɺͽṹ�����Ƶ��л�������������ͨʽΪ��CnH2n+2����n=4ʱ�����Ӷ��鿪ʼ����ͬ���칹�塣
��3��BΪ���������±�����ɫ����������ζ��Һ�����ܶȱ�ˮС��������ˮ���ж����������к��в����ͼ���һ�������¿��������������ӳɷ�Ӧ������̼̼˫��������������ʹ���Ը��������Һ����ˮ����ɫ�����ڢ���ȷ��
��4����Ũ���������£�����Ũ���ᷢ��������Ӧ��ȡ����Ӧ����������������ѧ����ʽΪ��![]() ��
��
��5��CΪCH3CH2OH����Է�������Ϊ4��EΪCH2=CH-COOH����Է�������Ϊ72�����߷���������Ӧ����ȡ����Ӧ��������Է�������Ϊ100�������仯ѧ����ʽΪ��CH2=CH-COOH��C2H5OH ![]() CH2=CH-COOC2H5��H2O��
CH2=CH-COOC2H5��H2O��

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�����Ŀ�����������£���һԪ��HA����Һ��KOH��Һ�������ϣ����Ի�Ϻ���Һ������仯����ʵ���������±���
ʵ����� | ��ʼŨ�ȣ���mol��L��1�� | ��Ӧ����Һ��pH | |
c��HA�� | c��KOH�� | ||
�� | 0.1 | 0.1 | 9 |
�� | x | 0.2 | 7 |
��ش�
��1��HA��Һ��KOH��Һ��Ӧ�����ӷ���ʽΪ________��
��2��ʵ������Ӧ�����Һ����ˮ�������c��OH������________mol��L��1��x________0.2mol��L��1����������������������������
��3�����й���ʵ������Ӧ�����Һ˵������ȷ����________������ĸ����
a����Һ��ֻ����������ƽ��
b����Һ�У�c��A������c��HA����0.1mol��L��1
c����Һ�У�c��K������c��A������c��OH������c��H����
������֪2H2��g����O2��g����2H2O��1�� ��H����572kJ��mol��1��ij����ȼ�ϵ�������ɶ��ʯī��Ϊ�缫��KOH��ҺΪ�������Һ��
��4��д���õ�ع���ʱ�����ĵ缫��Ӧʽ________��
��5����������ȼ�ϵ��ÿ�ͷ�228.8kJ����ʱ��������1molҺ̬ˮ����õ�ص�����ת����Ϊ________��