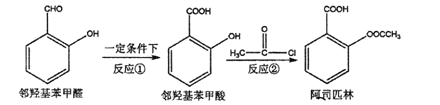
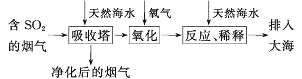
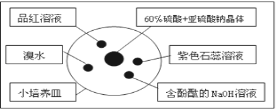
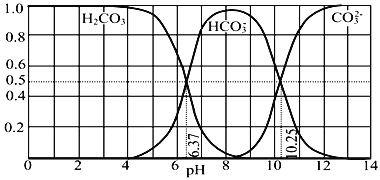
��Ŀ����
����Ŀ����Ҫ��ش��������⣺
(1)��![]() H��
H��![]() H��
H��![]() H��
H��![]() Mg��
Mg��![]() Mg��
Mg��![]() Cu�й���______��Ԫ�أ�______��ԭ��
Cu�й���______��Ԫ�أ�______��ԭ��
(2)���и�����:��16O2��18O2����H2��D2����1735Cl��1737Cl ����1H218O��2H216O����ͬλ�ص���___________________
(3)���������У�H2O2��KCl��Na2SO4��Na2O2��HCl��O2; ����ֻ�����Ӽ�����________ ��ֻ�����Լ�����______ ���Ⱥ����Ӽ��ֺ����Լ�����_____���Ⱥ����Ӽ��ֺ��Ǽ��Լ�����_______
(4)д���������ʵĵ���ʽ��Na2O2 ��______________ H2O2 ��________________
(5)�õ���ʽ��ʾ���л�������γɹ���
H2S��__________________________________
KCl��_________________
���𰸡�3 6 �� KCl HCl Na2SO4 Na2O2 ![]()
![]()
![]()

![]()
��������
(1)![]() H��
H��![]() H��
H��![]() HΪ��Ԫ�ص�3�ֲ�ͬ���أ�
HΪ��Ԫ�ص�3�ֲ�ͬ���أ�![]() Mg��
Mg��![]() MgΪþԪ�ص�2�ֲ�ͬ���أ�
MgΪþԪ�ص�2�ֲ�ͬ���أ�![]() CuΪͭԪ�ص�һ�ֺ��أ��ʹ���3��Ԫ�أ�6��ԭ�ӣ��ʴ�Ϊ��3��6��
CuΪͭԪ�ص�һ�ֺ��أ��ʹ���3��Ԫ�أ�6��ԭ�ӣ��ʴ�Ϊ��3��6��
(2)��16O2��18O2������Ԫ���γɵĵ��ʣ��ʢٲ�ѡ��
��H2��D2������Ԫ���γɵĵ��ʣ��ʢڲ�ѡ��
��1735Cl��1737Cl����Ԫ�صIJ�ͬ���أ���������ͬ����������ͬ����Ϊͬλ�أ��ʢ�ѡ��
��1H218O��2H216O����������Ԫ����ɵ�ˮ���ӣ��ʢܲ�ѡ��
�ʴ�Ϊ���ۣ�
(3)H2O2���м��Թ��ۼ����Ǽ��Թ��ۼ���KCl�������Ӽ���Na2SO4�������Ӽ������Թ��ۼ���Na2O2�������Ӽ����Ǽ��Թ��ۼ���HCl���м��Թ��ۼ���O2ֻ���Ǽ��Թ��ۼ���
��ˣ�ֻ�����Ӽ�����KCl��ֻ�����Լ�����HCl���Ⱥ����Ӽ��ֺ����Լ�����Na2SO4���Ⱥ����Ӽ��ֺ��Ǽ��Լ�����Na2O2���Ⱥ����Լ��ֺ��Ǽ��Լ�����H2O2���ʴ�Ϊ��KCl��HCl��Na2SO4��Na2O2��
(4)H2O2������Hԭ�Ӻ�Oԭ���γɹ��ۼ����ǹ��ۻ��������ʽΪ��![]() ��Na2O2�������Ӻ��������ӹ��ɵ����ӻ��������ʽΪ
��Na2O2�������Ӻ��������ӹ��ɵ����ӻ��������ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(5)H2S�ǹ��ۻ��������ʽ�γɵĹ��̣�![]() ���Ȼ��������ӻ�������ӻ�������������д����ɼ����Բ�����[ ]����������������д����ɺ͵��Լ��ɣ��Ȼ��صĵ���ʽΪ
���Ȼ��������ӻ�������ӻ�������������д����ɼ����Բ�����[ ]����������������д����ɺ͵��Լ��ɣ��Ȼ��صĵ���ʽΪ
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() ��
��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�����Ŀ�����ײ���һֱ�������о�����Ҫ���⣬��������Fe�۱�������г�ǿ�Ĵ��ԣ���Ч���Ե����������ʡ�
I��ʵ���Ҳ������ԭ���Ʊ�����Fe����������ͼ��ʾ��
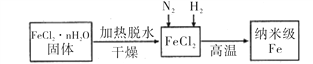
��1������Fe��ϡ���ᷴӦ�����ӷ���ʽΪ_______________________________��
��2����ν�FeCl2��nH2O���������ˮ�Ƶ���ˮFeCl2 _____________________________________(�ü�Ҫ��������)��
��3����������Fe�Ļ�ѧ����ʽΪ______________________________________��
II���������ϣ��ڲ�ͬ�¶��£�����Fe����ˮ������Ӧ�Ĺ�����ﲻͬ���¶ȵ���570��ʱ����FeO������570��ʱ����Fe3O4����ͬѧ����ͼ��װ����ʾ��������Fe����ˮ������Ӧ��ʵ�飬��ͬѧ��ͼ����ʾ��װ�ý�������Fe����ˮ�����ķ�Ӧ����֤���

��4����װ��������Fe����ˮ������Ӧ�Ļ�ѧ����ʽ�� ______________________��
��5����װ��������a������Ϊ_______________________��
��6����ͬѧΪ̽��ʵ��������Թ��ڵĹ������ʳɷ֣�����������ʵ�飺
ʵ�鲽�� | ʵ����� | ʵ������ |
I | ����Ӧ��õ��ĺ�ɫ��ĩX(�ٶ�Ϊ���ȵ�)��ȡ������������һ�Թ��У������������ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ��dz��ɫ�����������ݲ��� |
II | ��ʵ��I�õ�����Һ�еμӼ���KSCN��Һ���� | ��Һû�г��ֺ�ɫ |
��������ʵ�飬��ͬѧ��Ϊ�������·�Ӧ�Ĺ������ΪFeO��
��ͬѧ��Ϊ��ͬѧ�Ľ��۲���ȷ������������______(�ü�Ҫ��������)��
��7����ͬѧ��ȡ5.60gFe�ۣ�����װ��Ӧһ��ʱ���ֹͣ���ȡ����Թ��ڵĹ��������ڸ���������ȴ�Ƶ�����Ϊ6.88g����ͬѧʵ���Ĺ������������������������Ϊ________(���������λ��Ч����)��