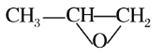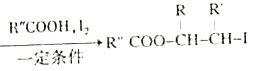��Ŀ����
����Ŀ��ͭ���仯�����������������й㷺Ӧ��
(1)��ҵ���Ի�ͭ��(CuFeS2)Ϊԭ�ϣ����û�������������ͭ���м���̻ᷢ����Ӧ:2Cu2O+Cu2S![]() 6Cu+SO2���÷�Ӧ����������________����֤��Ӧ������������SO2�ķ�����___________��
6Cu+SO2���÷�Ӧ����������________����֤��Ӧ������������SO2�ķ�����___________��
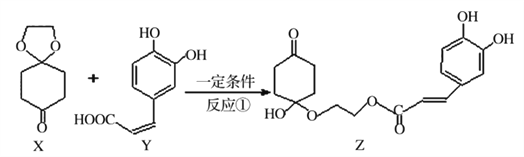
(2)������ͭ˿����������ϡ�����У��¶ȿ�����50�棬����H2O2����Ӧһ��ʱ������µ�60�棬�ٷ�Ӧһ��ʱ�����Ƶ�����ͭ���¶ȿ�����50�桫60�������ԭ����˼ӿ췴Ӧ�����⣬����___________����CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ���ȣ�����CuC1������д������CuCl�����ӷ���ʽ______________��
(3)��ʽ̼��ͭ���л��������̻���������ϡ�ũҩ�������й㷺��Ӧ�á�ij�����Ի�ͭ��(��Ҫ�ɷ�ΪCu2S��������Fe2O3��SiO2������)Ϊԭ���Ʊ���ʽ̼��ͭ����������:
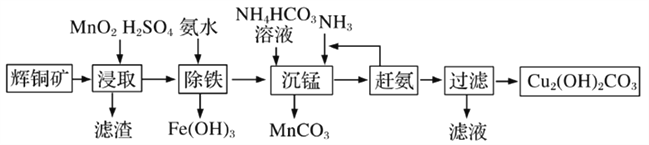
�ٻ�ͭ���ڽ�ȡǰҪ��������飬����ĺô���___________��
�ڽ�ȡ�����еõ�һ�ֵ��ʣ�д����ȡʱ��Ҫ��Ӧ�Ļ�ѧ����ʽ___________��
��д�������̡���Ӧʱ���ӷ���ʽ___________��
�ܡ���������һ����Ӧ��25����У����백ˮ������ҺpHΪ4����Һ��ͭ�������Ũ�Ȳ�����_________mol/L��(��֪Ksp[Cu(OH)2]=2.2��10-20)
���𰸡� Cu2S��Cu2O ������ͨ��Ʒ����Һ����Һ��ɫ�����Ȼָ�ԭɫ ͬʱ��ֹH2O2�ֽ� 2Cu2++2Cl-+SO32-+H2O![]() 2CuCl��+ SO42-+2H+ ����߽�ȡ���ʺͽ�ȡ�� Cu2S+2MnO2 +4H2SO4= 2CuSO4+S+2MnSO4+4H2O Mn2++HCO3-+NH3 = MnCO3��+NH4+ 2.2
2CuCl��+ SO42-+2H+ ����߽�ȡ���ʺͽ�ȡ�� Cu2S+2MnO2 +4H2SO4= 2CuSO4+S+2MnSO4+4H2O Mn2++HCO3-+NH3 = MnCO3��+NH4+ 2.2
����������1��2Cu2O+Cu2S![]() 6Cu+SO2�����÷�Ӧ��ͭ�Ļ��ϼ���+1�۱�Ϊ0�ۣ�ͭԪ���ڸ÷�Ӧ�еõ��ӻ��ϼ۽��ͣ����Ը÷�Ӧ�е���������Cu2O��Cu2S��SO2����Ư���ԣ���ʹƷ����Һ��ɫ�����Ȼָ�ԭɫ�����Խ�����ͨ��Ʒ����Һ��Һ��ɫ�����Ȼָ�ԭɫ��
6Cu+SO2�����÷�Ӧ��ͭ�Ļ��ϼ���+1�۱�Ϊ0�ۣ�ͭԪ���ڸ÷�Ӧ�еõ��ӻ��ϼ۽��ͣ����Ը÷�Ӧ�е���������Cu2O��Cu2S��SO2����Ư���ԣ���ʹƷ����Һ��ɫ�����Ȼָ�ԭɫ�����Խ�����ͨ��Ʒ����Һ��Һ��ɫ�����Ȼָ�ԭɫ��
��2������ͭ˿����������ϡ��������ͭ˿��ϡ�����Ӧ��������H2O2������˫��ˮ����ǿ�������������������¿���ͭ�����ɶ���ͭ��������Ӧ�����ӷ���ʽΪ��Cu+2H++H2O2=Cu2++2H2O����ΪH2O2�ڽϸ��¶�ʱ���ֽ��������¶ȿ�����50��-60�������Է�ֹH2O2�ֽ�����CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ����������CuCl������ͭԪ�صĻ��ϼ۽�������SO32-��Cu2+����ΪSO42-����Ӧ���Cu2+��Cl-��SO32-�⣬����H2O��������H+�����ݵ�ʧ�����غ��ԭ���غ���ƽ����2Cu2++2Cl-+SO32-+H2O![]() 2CuCl��+ SO42-+2H+�ʴ�Ϊ��ͬʱ��ֹH2O2�ֽ���2Cu2++2Cl-+SO32-+H2O
2CuCl��+ SO42-+2H+�ʴ�Ϊ��ͬʱ��ֹH2O2�ֽ���2Cu2++2Cl-+SO32-+H2O![]() 2CuCl��+ SO42-+2H+��
2CuCl��+ SO42-+2H+��
��3�������ʱ��ͨ�������ʯ���������¶Ȼ��߽��н�����������Ũ�ȣ���������߽�ȡ���ʣ��ʴ�Ϊ����߽�ȡ���ʣ�
�ڷ�Ӧ���Ƕ������̡���ͭ�����ᣬ��������S������ͭ�������̣���Ӧ�ķ���ʽΪ2MnO2+Cu2S+4H2SO4=S��+2CuSO4+2MnSO4+4H2O���ʴ�Ϊ��2MnO2+Cu2S+4H2SO4=S��+2CuSO4+2MnSO4+4H2O��
�ۡ����̡���Mn2+�����̣���Ҫ��ʹMn2+���ɳ���MnCO3����Ӧ�����ӷ���ʽΪMn2++HCO3-+NH3 = MnCO3��+NH4+ ���ʴ�Ϊ��Mn2++HCO3-+NH3 = MnCO3��+NH4+��
�ܸ���pHΪ4����֪��c(OH-)=10-10mol/L���ٸ���Ksp[Cu(OH)2]֪��c(Cu2+)=Ksp/[c2(OH-)]=![]() mol/L=2.2mol/L�����Դ�Ϊ2.2��
mol/L=2.2mol/L�����Դ�Ϊ2.2��

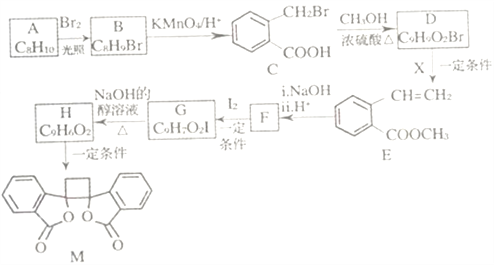
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
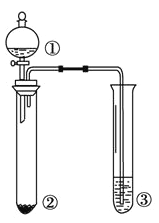
Сѧ��10����Ӧ����ϵ�д�����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | Ũ��ˮ | NaBr | ����KI��Һ | �����ԣ�Cl2>Br2>I2 | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | Br2�ı���Һ | ��м | AgNO3��Һ | �����嵥���������������� ����ȡ����Ӧ | |
D | ���� | Na2SO3 | KMnO4��Һ | SO2��ʹKMnO4��Һ��ɫ |
A. A B. B C. C D. D