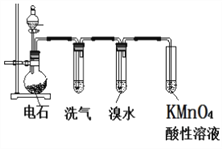
��Ŀ����
����Ŀ�������dzµ��㡱������Ϊ�����������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⡣

(1)��ϩ��ȡ�Ҵ��Ļ�ѧ����ʽ��__________________________��
(2)д����ȡ���������Ļ�ѧ��Ӧ����ʽ��________________________��
(3)Ũ��������ã�_______________________________��
(4)����̼������Һ����Ҫ������________________________________________��
(5)װ���е���Ҫ�ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ����__________��
(6)��Ҫ���Ƶõ������������������Ӧ�ò��õ�ʵ�������___________________��
(7)����ʵ��ʱ����ʱ��Ҫ��ʢ�������������Թ�����뼸�����Ƭ����Ŀ����________��
���𰸡� CH2=CH2+H2O![]() CH3CH2OH CH3COOH��CH3CH2OH
CH3CH2OH CH3COOH��CH3CH2OH![]() CH3COOC2H5��H2O ��������ˮ�� ��ӷ����������ᷴӦ��ʹ֮ת��Ϊ����������ˮ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ��������� ��ֹ���� ��Һ ��ֹ�Թ���Һ�屩��
CH3COOC2H5��H2O ��������ˮ�� ��ӷ����������ᷴӦ��ʹ֮ת��Ϊ����������ˮ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ��������� ��ֹ���� ��Һ ��ֹ�Թ���Һ�屩��
��������(1)��ϩ������ˮ�ӳ���ȡ�Ҵ�����Ӧ�Ļ�ѧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O
CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O![]() CH3CH2OH��
CH3CH2OH��
(2)������Ӧ�ı���Ϊ�����ǻ��������⣬�������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�Ӧ����ʽΪ��CH3COOH+CH3CH2OH![]() CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
(3)�������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ�����ʴ�Ϊ����������ˮ����
(4)�Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ������ʴ�Ϊ���к����ᡢ�ܽ��Ҵ������������������ܽ�ȣ�
(5)���ܲ��ܲ�����Һ�У�����Ҫ���ڱ���̼������Һ��Һ���ϣ�����Һ���¿��ܷ����������ʴ�Ϊ����ֹ������
(6)������������ʱ�Ƚ�ʢ�л������Թܳ�����ñ���̼������Һ�кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У��ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����÷ֲ��ȡ�ϲ�������������ʴ�Ϊ����Һ��
(7)Һ�����Ҫ�����Ƭ�������������ģ��ɷ�ֹ��Һ���У��ʴ�Ϊ����ֹ���С�

 �Ķ��쳵ϵ�д�
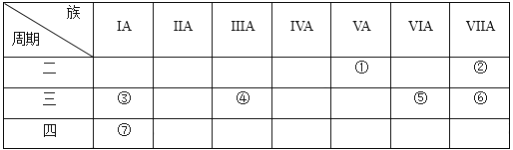
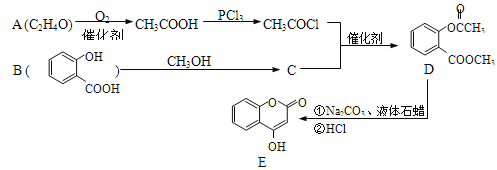
�Ķ��쳵ϵ�д�����Ŀ�������A��B��C���������й���Ϣ��
A | B | C |
����ʹ������Ȼ�̼��Һ��ɫ�� �ڱ���ģ��Ϊ��
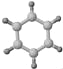
| ��ƽ���ͽṹ �ڹ�ģ��Ϊ�� | ����ʹ������Ȼ�̼��Һ��ɫ�� ��1mol����2molH2��һ�������·�Ӧ����A �۱���ģ��Ϊ�� |
|
ͼ2 |
���ݱ�����Ϣ�ش��������⣺
��1��д����A��ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽ_____________________��
��2����B����̼̼�����е��ص���_______________________
��3����ͼ1����B��Һ����ȡ�屽��װ�ã��Իش�
��a��װ��C�е�����___________________________________��
��b��װ��B������__________________________________��
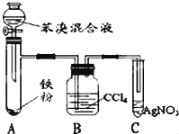
��4����ͼ2����ȡ��C��װ�ã��Իش�
��a��Ϊ�˿���������Ȳ�����ʣ���Һ©����ʢ�ŵ�Һ����_______________��
��b������Һ�����ƿ�з�����Ӧ������Ȳ��д���˷�Ӧ�Ļ�ѧ����ʽ��_________��
��c����ʯ�к��������ʣ�����H2S,PH3�����壬Ϊ�˲�����Ȳ������ɸ��ţ�Ӧ�ó��ӣ�һ��ѡ��___________ϴ����