��Ŀ����
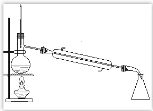
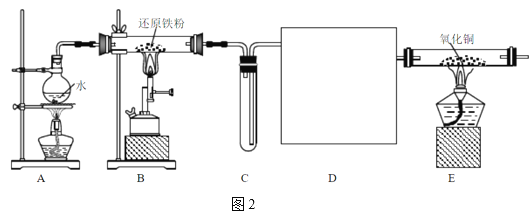
����Ŀ��ij����С����ʵ��������ͼ��ʾװ�ÿ�����ȡ������֤����ijЩ���ʣ�ͬʱ�ռ����������ĵ�������ش�
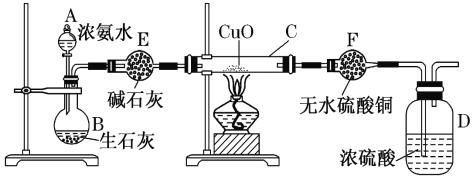
(1)д������ʯ�Һ�Ũ��ˮ��Ӧ�ư��Ļ�ѧ����ʽ____________________________�����ͷų�NH3��ԭ��_______________________________________________________��
(2)ʵ�����һ��ʱ�䣬�۲쵽Ӳ�ʲ������ں�ɫ����ͭ��ĩ��Ϊ��ɫ��ʢ��ˮ����ͭ�ĸ�����ڳ�����ɫ�����������ij������ܿڴ��ռ�������������ĵ�����������Щ����д����Ӳ�ʲ������ڷ�����Ӧ�Ļ�ѧ����ʽ��___________________________________��
(3)�����ij������ܿڴ��ռ���������ĵ������ռ�������________��
A���ſ����� B����ˮ�� C���������ռ�
(4) E�еļ�ʯ��________(������������������)����CaCl2��
(5) ��Ũ�����з���ͭƬ����ͭ��ʣ�࣬��ʼ��Ӧ�Ļ�ѧ����ʽΪ __________________��
(6) ����12.8 gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ��������NO��NO2 ����V L(��״��)����������������һ�������������ǡ�ñ�һ������NaOH��Һ��������������,��ͨ�����������ʵ�����_____��
���𰸡�NH3��H2O��CaO===Ca(OH)2��NH3�� ��ʯ����ˮ��Ӧ�ų������ȣ���ʹNH3��H2O���ȷֽ�����NH3���������¶����ߣ�NH3���ܽ�ȼ�С��ʹNH3��ˮ���ݳ� 3CuO��2NH3![]() 3Cu��3H2O��N2 C ���� Cu��4HNO3(Ũ)===Cu(NO3)2��2NO2����2H2O 0.1 mol
3Cu��3H2O��N2 C ���� Cu��4HNO3(Ũ)===Cu(NO3)2��2NO2����2H2O 0.1 mol
��������
��1����ʯ���백ˮ�е�ˮ��Ӧ�����������ƣ�ͬʱ�ų������ȣ���ʹNH3��H2O���ȷֽ�����NH3���������¶�����NH3���ܽ�ȼ�С��ʹNH3��ˮ���ݳ���
��2�����ȵ�Ӳ�ʲ������ں�ɫ����ͭ��ĩ��Ϊ��ɫ��˵���е���ͭ���ɣ�ʢ��ˮ����ͭ�ĸ�����ڳ�����ɫ��˵����ˮ���ɣ������ij������ܿڴ��ռ�������������ĵ������÷�Ӧ�Ļ�ѧ����ʽΪ3CuO��2NH3![]() 3Cu��3H2O��N2��
3Cu��3H2O��N2��
��3����N2���ܶ���������ܶ���������Բ������ſ����ռ�������Ҫ�õ������N2��Ҳ��������ˮ���ռ��������������ռ���
��4�������Ȼ������백����Ӧ�����Բ��ܰѼ�ʯ�һ�Ϊ�Ȼ��ƣ�
��5��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪCu��4HNO3(Ũ)=Cu(NO3)2��2H2O��2NO2����
��6�����ݵ�ʧ�����غ���м��㡣
��1����ʯ���백ˮ�е�ˮ��Ӧ�����������ƣ�ͬʱ�ų������ȣ���ʹNH3��H2O���ȷֽ�����NH3���������¶�����NH3���ܽ�ȼ�С��ʹNH3��ˮ���ݳ����ʷ�Ӧ�Ļ�ѧ����ʽΪ��NH3��H2O��CaO=Ca(OH)2��NH3�����ʴ�Ϊ��NH3��H2O��CaO===Ca(OH)2��NH3������ʯ����ˮ��Ӧ�ų������ȣ���ʹNH3��H2O���ȷֽ�����NH3���������¶����ߣ�NH3���ܽ�ȼ�С��ʹNH3��ˮ���ݳ���
��2�����ȵ�Ӳ�ʲ������ں�ɫ����ͭ��ĩ��Ϊ��ɫ��˵���е���ͭ���ɣ�ʢ��ˮ����ͭ�ĸ�����ڳ�����ɫ��˵����ˮ���ɣ������ij������ܿڴ��ռ�������������ĵ������÷�Ӧ�Ļ�ѧ����ʽΪ3CuO��2NH3![]() 3Cu��3H2O��N2���ʴ�Ϊ��3CuO��2NH3
3Cu��3H2O��N2���ʴ�Ϊ��3CuO��2NH3![]() 3Cu��3H2O��N2��
3Cu��3H2O��N2��
��3����N2���ܶ���������ܶ���������Բ������ſ����ռ�������Ҫ�õ������N2��Ҳ��������ˮ���ռ��������������ռ����ʴ�Ϊ��C��
��4��װ��E��ʢ�ŵ��Ǽ�ʯ�ң������������հ����е�ˮ������ˮ������ˮ����ͭ���ó�����ɫ����������ˮ����ͭ��������ͭ�Ͱ�����Ӧ�Ƿ���ˮ���ɣ������Ȼ������백����Ӧ�����Բ��ܰѼ�ʯ�һ�Ϊ�Ȼ��ƣ��ʴ�Ϊ�����ܡ�
��5��ͭ��Ũ���Ὺʼ��Ӧ�Ļ�ѧ����ʽΪCu��4HNO3(Ũ)=Cu(NO3)2��2H2O��2NO2�����ʴ�Ϊ��Cu��4HNO3(Ũ)=Cu(NO3)2��2NO2����2H2O��
��6����Ӧ���ĵ�ͭ�����ʵ���Ϊ12.8g��64g/mol=0.4mol��0.1molͭ��ȫ��Ӧʧȥ0.4mol���ӣ����ݵ����غ㣬�����õ��ĵ�����ͭʧȥ�ĵ���һ����ȣ����������������ʵ���Ϊ��0.4mol/4=0.1mol����ͨ�����������ʵ�����0.1mol���ʴ�Ϊ��0.1mol��

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�