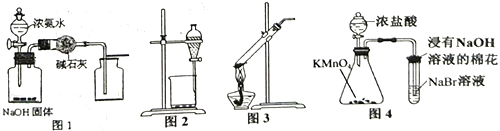
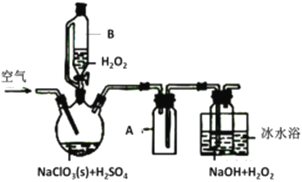
��Ŀ����
����Ŀ����ͼװ����,��U�ιܵײ�ʢ��CCl4,�ֱ���U�ι�����С�ĵ��뱥��ʳ��ˮ��ϡ������Һ,��ʹa��b����Һ����ƽ,Ȼ��ֱ����ϲ�������˿������,�ܷ��,����һ��ʱ���,�����й������д������

A. ��˿�������ĸ�ʴ����:a < b
B. a��b������ͬ�ĵ缫��ӦʽΪFe-2e-==Fe2+
C. һ��ʱ���,a��Һ�����b��Һ��
D. ����˿�е�̼��a��b�����ֱ���ԭ��صĸ���������
���𰸡�D
�����������������A��b����������Һ��a����������Һ������b�����������ⸯʴ��a�˷���������ʴ�������ⸯʴ���ʴ���������ʴ���ʣ���ȷ��B��a��b������Fe������������������Ӧ��ʧȥ���������������ӣ���ȷ��C����Ϊa����������ʴ��b�������ⸯʴ������b�˵�ѹǿ����a�˵�ѹǿ��С��a��Һ�����b��Һ�棬��ȷ��D�������е�̼����������������ѡD��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ