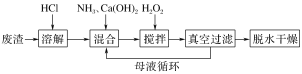
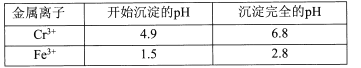
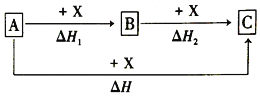
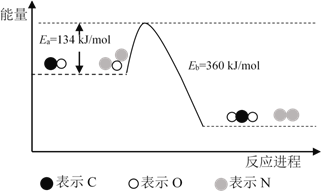
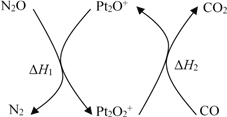
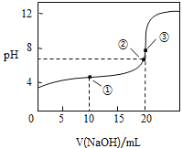
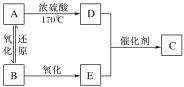
��Ŀ����
����Ŀ����֪B���Է���������Ӧ��D�������ˮ���Ĵ������C���������ζ����������ͼʾ���ش��������⣺
��1��A�ĽṹʽΪ________��
��2��B�й����ŵ�������________��
��3��D��E����C�ķ�Ӧ��������ɫ��ѧ����ѧ����ʽΪ___________
��4������˵����ȷ����________��
A ʯ���ѻ�������ȡD
B �������Ȼ�����ҺϴȥA��C��E�е�A��E
C D��E��ӦҲ��������C��ͬ���칹�壬��E��ͬϵ��
D B��A�ķ�Ӧ���ͣ�Ҳ�����Ǽӳɷ�Ӧ
���𰸡�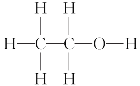 ȩ�� CH2=CH2��CH3COOH
ȩ�� CH2=CH2��CH3COOH![]() CH3COOCH2CH3 C��D
CH3COOCH2CH3 C��D
��������
D�������ˮ���Ĵ��������Ϊ��ϩ����AΪ�Ҵ���BΪ��ȩ��EΪ����Ӷ��ó�CΪ����������
��1��AΪ��ϩ���ɴ˿�д����ṹʽ��
��2��BΪ��ȩ���ɵó�������š�
��3��D��E����C�ķ�Ӧ��������ɫ��ѧ����Ӧ����Ϊ����������
��4��A.ʯ���ѽ������ȡD��
B. �����ñ���̼������ҺϴȥA��C��E�е�A��E
C. D��E��ӦҲ��������C��ͬ���칹�壬��E��ͬϵ���Ϊ�
D. B��A�ǽ�ȩ��ת��Ϊ���ǻ���
�����Ϸ�������ȷ��A��B��C��D��E�ֱ�Ϊ�Ҵ�����ȩ��������������ϩ�����ᡣ
��1��AΪ�Ҵ�����ṹʽΪ ����Ϊ��
������ ��
��
��2��BΪ��ȩ��������ŵ�������ȩ������Ϊ��ȩ����
��3��D��E����C�ķ�Ӧ��������ɫ��ѧ����ѧ����ʽΪCH2=CH2��CH3COOH![]() CH3COOCH2CH3����Ϊ:CH2=CH2��CH3COOH
CH3COOCH2CH3����Ϊ:CH2=CH2��CH3COOH![]() CH3COOCH2CH3��
CH3COOCH2CH3��
��4��A. ��֪DΪ��ϩ����ʯ���ѽ���A����ȷ��
B. A��C��E�ֱ�Ϊ�Ҵ����������������ᣬ��̼������Һϴ�ӣ�B����ȷ��
C. D��E��ӦҲ��������C��CH2=CH2��CH3COOH![]() CH3COOCH2CH3����������C��ͬ���칹�壺CH2=CH2��CH3COOH�D��CH3CH2CH2COOH��CH3COOH��CH3CH2CH2COOH��Ϊͬϵ�C��ȷ��
CH3COOCH2CH3����������C��ͬ���칹�壺CH2=CH2��CH3COOH�D��CH3CH2CH2COOH��CH3COOH��CH3CH2CH2COOH��Ϊͬϵ�C��ȷ��
D. B���Է���������Ӧ��Ϊ��ȩ��B��A������������Ҳ����Ϊ�ӳɷ�Ӧ��D��ȷ��
����CD��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�