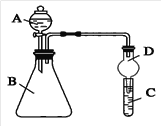
��Ŀ����
����Ŀ��ij����С������ͼװ�ý���ʵ�飬�Իش��������⣺

(1)����ʼʱ����K��a���ӣ���A���ĵ缫��ӦʽΪ____________________B����Fe����__________ ��ʴ(��������������������)
(2)����ʼʱ����K��b���ӣ�����˵����ȷ����________(�����)
����Һ��Na����A���ƶ���
�ڴ�A�����ݳ���������ʹʪ���KI������ֽ������
�۷�Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ�ȡ�
������״����B������2.24 L���壬����Һ��ת��0.2 mol����
�Ҵ�ʱװ�����ܷ�Ӧ�����ӷ���ʽΪ_____________________________________________________
(3)��С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼװ�õ���������Һ����ȡ������������������������ء�

�ٸõ��۵�������ӦʽΪ_________________________________________����ʱͨ�������ӽ���Ĥ��������________(������������С��������������)ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ���________(����A����B����C������D��)������
�ö��Ե缫������Ϊ1L��CuSO4��Һ����������������3.36L(���)����ʱ����������ͭ��Ũ��Ϊ______����Ҫ����Һ�ָ���ԭ״̬��Ҫ����________(��ѡ��)
A.CuSO4 B.CuO C.Cu(OH)2 D.Cu2(OH)2CO3
���𰸡�O2��2H2O��4e��===4OH�� ���� �ڢ� 2Cl����2H2O![]() 2OH����H2����Cl2�� 4OH����4e��===2H2O��O2��(��4H2O��4e��=== O2����4H+) С�� D 0.15mol/L C
2OH����H2����Cl2�� 4OH����4e��===2H2O��O2��(��4H2O��4e��=== O2����4H+) �� D 0.15mol/L C
��������
��1����ʼʱ����K��a���ӣ������ԭ��أ���Ϊ������AΪ������A�������õ����Ӳ���������������A�ĵ缫��ӦΪ��O2��2H2O��4e��===4OH����B����Fe����������ʴ��
��2���ٵ��ʱ�������������ƶ����ʢٴ���
��A������������ʹʪ��KI������ֽ�������ʢ���ȷ��
��������������������������������ͨ�������Ȼ���ɻָ���ԭŨ�ȣ��ʢ۴���
������״����B������2.24L��������2H++2e-=H2����֪ת��0.2mol���ӣ��ʢ���ȷ��
�ʴ�Ϊ���ڢܣ�
����K��b���ӣ�װ��Ϊ���أ���Ϊ������������ԭ��Ӧ�������ӵõ����������������缫����ʽΪ2H++2e-=H2�����ܵĵ缫��Ӧ�ǵ�ⱥ��ʳ��ˮ���缫��Ӧ����ʽΪ��2Cl����2H2O![]() 2OH����H2����Cl2����
2OH����H2����Cl2����
(3) �ٵ��ʱ��������ʧ���ӷ���������Ӧ����Һ�е����������ӵķŵ�����������������ӵķŵ���������������������������ʧ��������ˮ������4OH����4e��===2H2O��O2����4H2O��4e��=== O2����4H+���������������ӷŵ磬�������������������ƶ������������ӷŵ磬����������������ƶ�������ͨ����ͬ����ʱ��ͨ�������ӽ���Ĥ��������С��ͨ�������ӽ���Ĥ����������
�ʴ�Ϊ��4OH����4e��===2H2O��O2����4H2O��4e��=== O2����4H+������
�������������������ɣ�������D�ڵ�����
��ʯī�缫���CuSO4��Һ��������ӦΪ:4OH- -4e-=2H2O+ O2����
�����缫��ӦΪ:Cu2+ +2e- = Cu,2H++2e- = H2��,�ܷ�Ӧ���ӷ���ʽΪ:
2Cu2+ + 2H2O![]() 2Cu +4H++O2����������������״��������3.36Lʱ���������������ʵ���Ϊ:
2Cu +4H++O2����������������״��������3.36Lʱ���������������ʵ���Ϊ: ![]() = 0.15mol, ����ת��0.15mol
= 0.15mol, ����ת��0.15mol![]() 4=0.6mol, �������������ʵ���Ϊ:
4=0.6mol, �������������ʵ���Ϊ: ![]() = 0.15mol, ����ת��0.15mol
= 0.15mol, ����ת��0.15mol![]() 2=0.3mol,���ݵ�ʧ�����غ��֪��n(Cu2+)=
2=0.3mol,���ݵ�ʧ�����غ��֪��n(Cu2+)=![]() = 0.15mol������ͭ��Ũ��Ϊ0.15mol/L���ݴ˽��з���,����������������������Cu����������Ӧ����Cu(OH)2���ɻָ���ԭ״̬,��ѡC��
= 0.15mol������ͭ��Ũ��Ϊ0.15mol/L���ݴ˽��з���,����������������������Cu����������Ӧ����Cu(OH)2���ɻָ���ԭ״̬,��ѡC��

����Ŀ����֪ij���巴Ӧ��ƽ�ⳣ���ɱ�ʾΪK=c(CH3OCH3)c(H2O)/c2(CH3OH)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ����400����K=32��500����K=44����ش��������⣺
��1��д��������Ӧ�Ļ�ѧ����ʽ��_________________________________ ��
��2���÷�Ӧ����H _________0��
��3����֪���ܱ������У����ijʱ�̸���ֵ�Ũ�����£�
���� | CH3OH(g�� | CH3OCH3(g�� | H2O(g�� |
Ũ��/��molL-1�� | 0.54 | 0.68 | 0.68 |
�ٴ�ʱ�¶�400�棬��ijʱ������_______���������������ͬ����
�������¶�Ϊ�����꣬�Ը��¶���ƽ��̬�״����ʵ���nΪ�����꣬��ʱ��Ӧ����ͼ���λ����ͼ��____�㣬�Ƚ�ͼ��B��D��������Ӧ������Ӧ������B_______��D��������____��

��4��һ��������Ҫ��߷�Ӧ���ת���ʣ����Բ��õĴ�ʩ��___________��
a�������¶� b��������� c��ѹ�����������
d������ˮ������Ũ�� e����ʱ���������