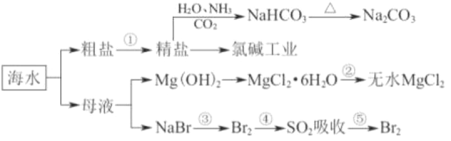
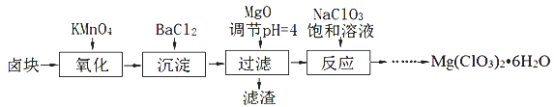
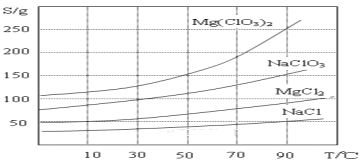
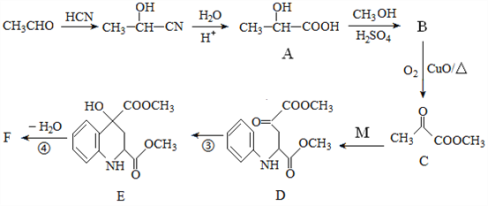
��Ŀ����
����Ŀ�������Ǹ�[(CH3COO)2Cr��H2O��Ϊש��ɫ���壬��������ˮ���������ᣬ����������������������ռ���һ���Ʊ����������ڷ����ϵ�����ý���п����ԭ���������۸���ԭΪ���۸������۸������������Һ���ü����Ƶô����Ǹ���ʵ��װ����ͼ��ʾ���ش��������⣺

��1��ʵ������������ˮ���辭��к�Ѹ����ȴ��Ŀ����_________������a��������_______��
��2��������п�����Ȼ�����������c�У�������������ˮ����ͼ���Ӻ�װ�ã���K1��K2���ر�K3��
��c����Һ����ɫ��Ϊ����ɫ���÷�Ӧ�����ӷ���ʽΪ_________��
��ͬʱc��������������������������_____________��
��3����K3���ر�K1��K2��c������ɫ��Һ����d����ԭ����________��d������ש��ɫ������Ϊʹ����������������룬����õIJ�����___________��_________��ϴ�ӡ����
��4��ָ��װ��d���ܴ��ڵ�ȱ��______________��
���𰸡� ȥ��ˮ���ܽ��� ��Һ�����Һ��©�� Zn+2Cr3����Zn2��+2Cr2�� �ų�c�п��� c�в���H2ʹѹǿ���ڴ���ѹ ����ԡ����ȴ ���� ������ϵ������ʹ�����Ǹ�������Ӵ�
����������������������Һ��п��Cr3����ԭΪCr2����ͬʱ���������ž�װ���еĿ�����ֹ���������ɵ���������c��ѹǿ���������ɵ�CrCl2ѹ��dװ�÷�����Ӧ���ݴ˽��
��⣺��1�����ڴ����Ǹ��ױ�������������Ҫ�����ܱ����������Ӵ������ʵ������������ˮ������к�Ѹ����ȴ��Ŀ����ȥ��ˮ���ܽ������������������֪����a�Ƿ�Һ�����Һ��©����
��2����c����Һ����ɫ��Ϊ����ɫ��˵��Cr3����п��ԭΪCr2������Ӧ�����ӷ���ʽΪZn+2Cr3����Zn2��+2Cr2����
��п���������ᷴӦ��������������װ���к��п�����������Cr2���������������������ų�c�п�����
��3����K3���ر�K1��K2������п���������ᷴӦ��������������c��ѹǿ��������c������ɫ��Һ������dװ�ã�������Ʒ�Ӧ�����������Ϣ��֪�����Ǹ�������ˮ��ˮ������Ϊʹ����������������룬��Ҫ��ȡ�IJ����ǣ���ԡ����ȴ�����ˡ�ϴ�ӡ����
��4������dװ���dz�����ϵ�����װ�õ�ȱ���ǿ���ʹ�����Ǹ�������Ӵ���������ʹ��Ʒ������

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� ��Ȥ����¹�֪��ϵ�д�
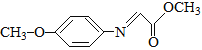
��Ȥ����¹�֪��ϵ�д�����Ŀ�����Ʊ����ǿ�ѧ�о�����Ҫ��������ͬ����������������ϡ����ֱ������������·�����Ӧ�����з�Ӧ����������
A | B | C | D | |
t/�� | 10 | 10 | 40 | 40 |
c(H2SO4 )/(mol/L) | 1 | 3 | 1 | 3 |
A. AB. BC. CD. D
����Ŀ��Ϊ̽��Na2SO3��Һ���������ڰ�ɫ��ΰ��a��b��c��d�ĸ������е���Na2SO3��Һ���ٷֱ�μ���ͼ��ʾ���Լ���

���й���ʵ������Ľ��ͻ������ȷ����
ѡ�� | ʵ������ | ���ͻ���� |
A | a������������ | Na2SO3��H2O2һ����������Ӧ |
B | b�м�ϡH2SO4��Ų�������ɫ���� | SO32��S2��������һ�����ܴ������� |
C | c�м�BaCl2��Һ�������ɫ�����Һ�ɫ��ȥ | Ba2++ SO32 == BaSO3����ʹSO32ˮ��ƽ�������ƶ�����ɫ��ȥ |
D | d�в�����ɫ���� | ԭNa2SO3��Һ�к���SO42 |
A. A B. B C. C D. D