��Ŀ����
����Ŀ����TiCl3�ı���Һ��ͨ��HCl�����ͣ��ټ�������������ɫ���壬������������ѣ�ֱ��ͨ��HCl�õ�������ɫ���壬��֪���־������ʽ��ΪTiCl3��6H2O����λ������6�������ֱ�ȡ0.01mol���־�����ˮ��Һ���ù���AgNO3��������ɫ����õ��İ�ɫ��������Ϊ��ɫ����õ�����������![]() ���������й�˵������ȷ���ǣ� ��
���������й�˵������ȷ���ǣ� ��
A.����ɫ���������������Ӻ�ˮ���������ʵ���֮��Ϊ1��5
B.��ɫ���������Ļ�ѧʽΪ[Ti(H2O)6]Cl3
C.�������־���ķ���ʽ��ͬ�����ṹ��ͬ���������ʲ�ͬ
D.0.01mol��ɫ������ˮ��Һ�������AgNO3�������ɵõ�2.78g����
���𰸡�D
��������
������ЃȽ�ԭ�Ӳ��ܷ������룬���������ˮ��Һ���ܷ������룬�����ӿ����������ӷ�Ӧ�����Ȼ�����ɫ������ͨ�����������������ƶϳ������ӵĺ�����ԭ��ɫ�����ˮ��Һ��AgNO3��Һ��Ӧ�õ��İ�ɫ��������Ϊ��ɫ�����ˮ��Һ��Ӧ�õ�����������![]() �����־������ɽ�ΪTiCl3��6H2O��˵����ɫ�����������������ƶ��������ӣ�����ɫ������ֻ��2�������ƶ������ӣ�����һ����ԭ���γ���������Ϊ��Ϊ6��λ������������������ˮ������ѧʽΪ[TiCl��H2O��5]Cl2��H2O������ɫ����Ļ�ѧʽΪ[Ti��H2O��6]Cl3��
�����־������ɽ�ΪTiCl3��6H2O��˵����ɫ�����������������ƶ��������ӣ�����ɫ������ֻ��2�������ƶ������ӣ�����һ����ԭ���γ���������Ϊ��Ϊ6��λ������������������ˮ������ѧʽΪ[TiCl��H2O��5]Cl2��H2O������ɫ����Ļ�ѧʽΪ[Ti��H2O��6]Cl3��
A����ɫ�������ʽΪTiCl3��6H2O����λ����6����2�������ӣ���ѧʽΪ[TiCl��H2O��5]Cl2��H2O������ɫ���������������Ӻ�ˮ���������ʵ���֮��Ϊ1��5����A��ȷ��
B����ɫ�����к�3�������ӣ���λ����6����ɫ���������Ļ�ѧʽΪ[Ti(H2O)6]Cl3����B��ȷ��
C�����־������ʽ��ΪTiCl3��6H2O���ù���AgNO3��������ɫ����õ��İ�ɫ��������Ϊ��ɫ����õ�����������![]() ����������ͬ���ṹ��ͬ���������ʲ�ͬ����C��ȷ��
����������ͬ���ṹ��ͬ���������ʲ�ͬ����C��ȷ��
D��������ЃȽ�ԭ�Ӳ��ܷ������룬���������ˮ��Һ���ܷ������룬��ɫ�����к�3�������ӣ���ɫ����Ļ�ѧʽΪ[Ti(H2O)6]Cl3��0.01mol ��ɫ������ˮ��Һ�������AgNO3�������ɵõ�0.03mol����������Ϊm=nM=0.03mol��143.5g��mol��1=43.05g����D����
��ѡD��

����Ŀ���������Ǻϳ�������66����Ҫԭ��֮һ��ʵ���Һϳɼ������ԭ�����й��������£�
3![]() ��8HNO3��3
��8HNO3��3![]() ��8NO����7H2O
��8NO����7H2O
���� | ��Է������� | �ܶȣ�20�棩 | �۵� | �е� | �ܽ��� |
������ | 100 | 0.962 g/cm3 | 25.9�� | 160.8�� | 20��ʱ����ˮ���ܽ��Ϊ3.6g���ɻ������Ҵ����� |
������ | 146 | 1.360 g/cm3 | 152�� | 337.5�� | ��ˮ�е��ܽ�ȣ�15��ʱ1.44g��25��ʱ2.3g���������Ҵ��������ڱ� |
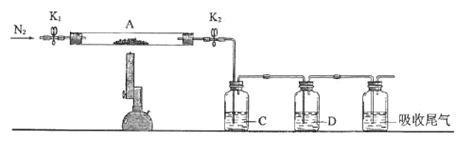
���������ͼװ�õ�������ƿ�м���16 mL 50%�����ᣨ�������ܶ�Ϊ1.310 g/cm3�����ټ���1��2����ʯ����Һ©����ʢ����5.4 mL��������

�����ˮԡ����������ƿ��50�����ң���ȥˮԡ�������μ�5��6�λ�������ҡ��������ƿ���۲쵽�к���ɫ����ų�ʱ�������μ�ʣ�µĻ�������ά�ַ�Ӧ�¶���60�桫65��֮�䡣
�����������ȫ��������������80�桫90��ˮԡ����Լ10 min��ע������¶ȣ���ֱ������ɫ��������Ϊֹ��
����������Ƚ���ӦҺ�����ձ��У������ˮԡ����ȴ�������������ˡ�ϴ�ӡ�������ء���ش��������⣺
��1��װ��b������Ϊ__________��ʹ��ʱҪ��_________�������Ͽ��������¿�����ͨ����ˮ����Һ©����ϸ֧��a��������________________��
��2��ʵ���У��Ƚ��¶�����������50�����ң�������������60�桫65��֮�䣬��������80�桫90�棬Ŀ����____________________��
��3����ʵ�����õ�50%���������ʵ���Ũ��Ϊ____________��ʵ���У����������������Ҫ�ɷ�ΪNO��NO2��������NaOH��Һ�����գ�����Ҫ��ӦΪNO+NO2+2NaOH == 2NaNO2+H2O������NaOH��Һ������Na2CO3��Һ���������ģ��������Ӧ��д��Na2CO3��Һ���յķ���ʽ��______________________________________��
��4��Ϊ�˳�ȥ���ܵ����ʺͼ��ٲ�Ʒ��ʧ���ɷֱ��ñ�ˮ��______ϴ�Ӿ��塣
��5��ͨ�������õ�����7.00 g����ʵ�����Ϊ__________(��ȷ��0.1��)��
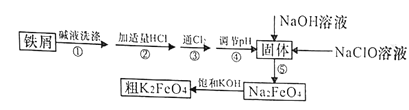
����Ŀ���Է���м(����������)�Ʊ��������(K2FeO4)����������ͼ��ʾ��
��֪��25��ʱ��һЩ�����������↑ʼ��������ȫ����ʱ��pH���±���ʾ��
M(OH)m | PH | |
��ʼ���� | ������ȫ | |
Fe (OH)3 | 2.53 | 2.94 |
Ni(OH)2 | 7.60 | 9.75 |
(1)K2FeO4����Ԫ�صĻ��ϼ�Ϊ________________��
(2)����Һϴ������Ŀ���dz�ȥ��м��������ۣ�ʵ��һ��ѡ��Na2CO3��Һ���ۣ�ѡ��Na2CO3��Һ���۵�ԭ����____________________________(�����ӷ���ʽ��ʾ)��
(3)����۷�����Ӧ�����ӷ���ʽΪ___________________��
(4)������ǽ�Fe(OH)3��������ΪNa2FeO4��ͬʱNaClOת��ΪNaCl��������1mol Na2FeO4����NaClO������Ϊ______g������ܵ���pH�ķ�Χ��_______��
(5)�õζ����ⶨ���ƴ�K2FeO4�Ĵ���(������KI����Ӧ)��ȡ0.220g��K2FeO4��Ʒ���������������ữ��KI��Һ����ַ�Ӧ����0.200mol��L��1Na2S2O3����Һ�ζ����ɵ�I2���ζ����ı���Һ�����Ϊ20.00mL���漰�ķ�Ӧ�У�FeO42����4I����8H����Fe2����2I2��4H2O��2S2O32����I2��S4O62����2I����
�ٵζ�ʱѡ�õ�ָʾ��Ϊ______���ζ��յ������Ϊ_____________��
�ڴ�K2FeO4�Ĵ���Ϊ_____________��