��Ŀ����
����Ŀ���������Һ������������Ӧ��ʮ�ֹ㷺���ش��������⣺
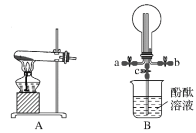
��1���Ȼ�����������ˮ������ԭ���ǣ������ӷ���ʽ��ʾ��___��
��2��ˮ�����ڹ�ҵ�Ͽ���ճ�ϼ�������NH4Cl��Һ�Ӵ�ʱ����ܿ����Ტ�ų��̼�����ζ�����壬��ԭ���ǣ������ӷ���ʽ��ʾ��___��
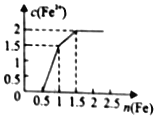
��3��Ũ�Ⱦ�Ϊ0.1mol��L��1�����и���Һ����HCl ��NaOH ��Na2CO3 ��CH3COONa ��NH4Cl ��H2SO4 ��KCl ��CH3COOH��pH��С���������˳��Ϊ___��
��4����֪��0.1mol��L��1NaHSO3 ��Һ�е���ʯ����Һ��죬��0.1mol��L��1NaHSO3��Һ������Ũ�ȴ�С��ϵΪ��___��
��5����mmol/L�Ĵ����nmol/L������������Һ�������Ϻ���Һ��pH��7������Һ��c(CH3COO-)+c(CH3COOH)=__mol/L��m��n�Ĵ�С��ϵ��m__n������>��������������<������
���𰸡�Fe3++3H2O![]() Fe(OH)3(����)+3H+ SiO32-+2NH4+=2NH3��+H2SiO3�� �ڣ��ۣ��ܣ��ߣ��ݣ��٣��� c(Na+)>c(HSO3-)> c(H+)>c(SO32-)>c(OH-)
Fe(OH)3(����)+3H+ SiO32-+2NH4+=2NH3��+H2SiO3�� �ڣ��ۣ��ܣ��ߣ��ݣ��٣��� c(Na+)>c(HSO3-)> c(H+)>c(SO32-)>c(OH-) ![]() mol/L ��
mol/L ��
��������
��1���Ȼ�������ˮ������ΪFe3+ˮ��ΪFe(OH)3�����Ե�ʣ�
��2���Ȼ����Һ��笠�����ˮ�������ԣ�
��3����������ε������ǿ�������жϣ�
��4��NaHSO3��Һ�����ԣ�˵����Һ��HSO3-����̶ȴ���ˮ��̶ȣ�
��5�����������غ�����c(CH3COO-)+c(CH3COOH)=![]() mol/L��
mol/L��
��1���Ȼ�������ˮ������ΪFe3+ˮ��ΪFe(OH)3�����Ե�ʣ�ԭ��ΪFe3++3H2O![]() Fe(OH)3(����)+3H+��Fe(OH)3�����ܹ��������ʣ��Ӷ�ʹˮ������
Fe(OH)3(����)+3H+��Fe(OH)3�����ܹ��������ʣ��Ӷ�ʹˮ������
��2���Ȼ����Һ��笠�����ˮ�������ԣ�ˮ����������Һ�������ӽ�����ɹ��ὺ������ᣬˮ������NH4Cl��Һ��Ӧ�����Ȼ��ơ����ᡢ���������ӷ�ӦΪSiO32-+2NH4+=2NH3��+H2SiO3����
��3��Ũ�Ⱦ�Ϊ0.1mol��L��1�����и���Һ����HCl ��ǿ����ʣ�pH=1��
��NaOH��ǿ����ʣ�pH=13��
��Na2CO3��ǿ�������Σ�����Һ�ʼ��ԣ�pH��7��
��CH3COONa��ǿ�������Σ�����Һ�ʼ��ԣ�pH��7������������ǿ��̼�ᣬ��pHС�ڢۣ�
��NH4Cl��ǿ�������Σ�笠�����ˮ���ʹ����Һ�������ԣ�pH��7����ˮ�������ģ�
��H2SO4��ǿ����ʣ�pH<1��
��KCl��ǿ��ǿ���Σ���Һ�����ԣ�pH=7��
��CH3COOH�����ᣬ����ȫ���룬7��pH��1��
�����⼸����ҺpH��С˳���Ǣڣ��ۣ��ܣ��ߣ��ݣ��ࣾ�٣��ޣ�
��4����֪��0.1mol��L��1NaHSO3 ��Һ�е���ʯ����Һ��죬��Һ�����ԣ�˵����Һ��HSO3-����̶ȴ���ˮ��̶ȣ�c(H+)����c(OH-)����0.1mol��L��1NaHSO3��Һ������Ũ�ȴ�С��ϵΪ��c(Na+)>c(HSO3-)> c(H+)>c(SO32-)>c(OH-)��
��5����m mol/L�Ĵ����n mol/L������������Һ�������Ϻ���Һ��pH=7�����������غ�����c(CH3COO-)+c(CH3COOH)=![]() mol/L����m=nʱ������Һǡ�÷�Ӧ���ɴ����ƣ���Һ��ʾ���ԣ���ʹ��Һ��pH=7��������Ũ��Ӧ���Դ�һЩ����m��n��
mol/L����m=nʱ������Һǡ�÷�Ӧ���ɴ����ƣ���Һ��ʾ���ԣ���ʹ��Һ��pH=7��������Ũ��Ӧ���Դ�һЩ����m��n��

����Ŀ������β���к���CO��NO���к����塣
(1)ͨ��NO�������ɼ������β����NO�ĺ����乤��ԭ����ͼ��ʾ����֪��![]() ���ڹ��������������ƶ���
���ڹ��������������ƶ���
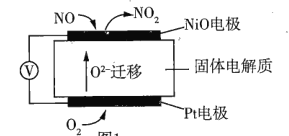
��NO�缫�Ϸ�������______(���������ԭ��)��Ӧ��
�����·�У������Ǵ�______(�NO����Pt��)�缫������
��Pt�缫�ϵĵ缫��ӦʽΪ______��
(2)һ�����ʹ�������NO��CO�ķ�Ӧ��![]() ����֪��������ıȱ��������߸÷�Ӧ�����ʣ�Ϊ����֤�¶ȡ������ıȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죬ijͬѧ���������ʵ�飬�����ʾ��
����֪��������ıȱ��������߸÷�Ӧ�����ʣ�Ϊ����֤�¶ȡ������ıȱ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죬ijͬѧ���������ʵ�飬�����ʾ��
ʵ���� |
| NO��ʼŨ��/( | CO��ʼŨ��/( | �����ıȱ����( |
�� | 280 |
|
| 82 |
�� | 280 |
|
| 124 |
�� | 350 | a |
| 82 |
�ٱ���a=______��
������֤�¶ȶԻ�ѧ��Ӧ����Ӱ�����ʵ��______(��ʵ�����)��
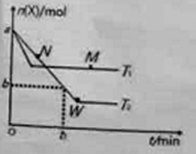
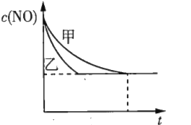
��ʵ��I��ʵ����У�NO�����ʵ���Ũ��![]() ��ʱ��t�ı仯������ͼ��ʾ�����б�ʾʵ����������______(��ס����ҡ�)��
��ʱ��t�ı仯������ͼ��ʾ�����б�ʾʵ����������______(��ס����ҡ�)��
(3)���ݻ��̶��ľ��������з�����Ӧ![]() ������˵���÷�Ӧ�Ѵﵽƽ��״̬����______(�����)��
������˵���÷�Ӧ�Ѵﵽƽ��״̬����______(�����)��
A.�������¶Ȳ��ٱ仯 B.�����ڵ�����ѹǿ���ֲ���
C.![]() D.�����ڻ��������ܶȱ��ֲ���
D.�����ڻ��������ܶȱ��ֲ���
����Ŀ���������ʵ���;���������������ʵ���
ѡ�� | ���� | ��; |
A | ������ԣ� | ��Ϊ�����������ӵ�������Ȼ���У���ʾ����й© |
B | ���� | װ����ֽ���У����ڸ�֬ʳƷ��װ���� |
C | KMnO4 | ���ݹ���������������ˮ���ı��ʼ� |
D | K2Cr2O7 | ���ھƼݼ�����У�����˾���Ƿ�Ƽ� |
A.AB.BC.CD.D