��Ŀ����
����Ŀ�������������ĿǰΪֹ������ǿ���������ô����������������������Ҫԭ������ϡ�������ϡ��������������Լ�����ϡ��ԭ�ϡ�
��1��д�����������ӵ���Χ�����Ų�ͼ��___���ڶ�����Ԫ���У���Al�Ļ�ѧ���������Ƶ�Ԫ�ط����ǣ�___��
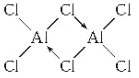
��2��ʵ����AlCl3�������Զ��۷���Al2Cl6����ʽ���ڣ���֪Al2Cl6������Al��Cl�������У�Ϊ�Ǽ��Է��ӣ�Al��Cl������8�����ȶ��ṹ����Al2Cl6������Alԭ�Ӳ�ȡ___�ӻ���Al2Cl6���ӵĽṹʽΪ��___��
��3����B��Ԫ�ؼ�����ͬ�������ڵ�����Ԫ�ص�һ��������С�����˳��Ϊ��___(��Ԫ�ط��ű�ʾ)��
��4����֪AlCl3���۵�Ϊ194�棬��������AlF3���۵�Ϊ1040�棬��ԭ��Ϊ��___��
��5����֪����������BN���侧���ṹ�����ڽ��ʯ����ͼ��ʾ������N����λ����___����Nԭ�Ӿ��������Bԭ�ӹ��ɵ����幹����___��

��ͼ��b��ԭ�ӵ�����Ϊ��![]() ��
��![]() ��0��д��aԭ�ӵ�����___���辧����B��Nԭ�Ӱ뾶�ֱ�Ϊpnm��qnm�������߳�Ϊrnm������aԭ��Ϊ�����γɵ���С������Ŀռ�������Ϊ___��д����ʽ���ɣ����ػ����辧���������B��Nԭ��֮��ľ���Ϊa nm��������ܶ�Ϊbg��cm��3�����ӵ�����Ϊ___mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
��0��д��aԭ�ӵ�����___���辧����B��Nԭ�Ӱ뾶�ֱ�Ϊpnm��qnm�������߳�Ϊrnm������aԭ��Ϊ�����γɵ���С������Ŀռ�������Ϊ___��д����ʽ���ɣ����ػ����辧���������B��Nԭ��֮��ľ���Ϊa nm��������ܶ�Ϊbg��cm��3�����ӵ�����Ϊ___mol��1(�ú�a��b�Ĵ���ʽ��ʾ)��
���𰸡�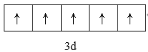 Be sp3�ӻ�
Be sp3�ӻ�  ��
�� B<Be<C AlCl3Ϊ���Ӿ��壬AlF3Ϊ���Ӿ��壬���AlF3���۵����AlCl3 4 ������ ��
B<Be<C AlCl3Ϊ���Ӿ��壬AlF3Ϊ���Ӿ��壬���AlF3���۵����AlCl3 4 ������ ��![]() ��
��![]() ��
��![]() ��
�� 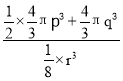 ��100%
��100% 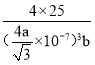
��������
��1�����ú��ع����Լ�����ԭ�����Խ���ԭ����з�����
��2�����ü۲���Ӷ��������ӻ���������з�����
��3�����õ�һ�����ܵĹ��ɽ��з�����
��4�����ò�ͬ�����۷е�ȽϽ��з�����
��5�����ݾ����Ľṹ���з�����
��1����Ԫ��Ϊ����Ԫ�أ�Fe3�������Ų�ʽΪ[Ar]3d5�����պ��ع��������ԭ����Fe3������Χ�����Ų�ͼΪ �����öԽ���ԭ������Ԫ���У���Al�Ļ�ѧ���������Ƶ�Ԫ�ط�����Be��
�����öԽ���ԭ������Ԫ���У���Al�Ļ�ѧ���������Ƶ�Ԫ�ط�����Be��
��Ϊ ��Be��
��Be��
��2������������Ϣ��Al2Cl6Ϊ�Ǽ��Է��ӣ�˵���ǶԳƽṹ��Al��Cl������8�����ȶ��ṹ��Al2Cl6�ṹʽΪ ��
�� ��Al2Cl6������1��Al��4��Cl������Alԭ�Ӳ�ȡsp3�ӻ���
��Al2Cl6������1��Al��4��Cl������Alԭ�Ӳ�ȡsp3�ӻ���
��Ϊsp3�ӻ��� ��
�� ��
��
��3��ͬһ����Ԫ�ش����ҵ�һ��������������A>��A����A>��A����˵�һ�����ܴ�С˳����B<Be<C��
��ΪB<Be<C��
��4��AlCl3���۵�Ϊ194�棬�����������Ƿ��Ӿ�����ص㣬��AlCl3Ϊ���Ӿ��壬AlF3���۵�Ϊ1040�棬��AlF3Ϊ���Ӿ��壬���AlF3���۵����AlCl3��
��ΪAlCl3Ϊ���Ӿ��壬AlF3Ϊ���Ӿ��壬���AlF3���۵����AlCl3��
��5�����ݾ����ṹ�������������е�ԭ������Χ��4����ԭ���γ�������ṹ����N����λ��Ϊ4����Nԭ�Ӿ��������Bԭ�ӹ���������ṹ���þ���Ϊԭ�Ӿ��壬a������������B�ľ�����Խ��ߵ�![]() �����a�������Ϊ��
�����a���������![]() ��
��![]() ��
��![]() ������aԭ��Ϊ�����γɵ���С�������Ǹþ�����
������aԭ��Ϊ�����γɵ���С�������Ǹþ�����![]() ���ṹ��
���ṹ��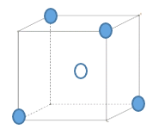 ����С�������Bԭ�Ӹ���Ϊ
����С�������Bԭ�Ӹ���Ϊ![]() ������С�������Nԭ�Ӹ���Ϊ1��ԭ�������
������С�������Nԭ�Ӹ���Ϊ1��ԭ�������![]() ��С�����������Ǿ��������
��С�����������Ǿ��������![]() ����С����������Ϊ
����С����������Ϊ![]() r3����ռ�������Ϊ
r3����ռ�������Ϊ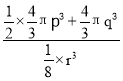 ��100%�����B��Nԭ��֮��ľ���Ӧ����Խ��ߵ�
��100%�����B��Nԭ��֮��ľ���Ӧ����Խ��ߵ�![]() ������Խ��ߵľ���Ϊ4a�����ݹ��ɶ������ó������ı߳�Ϊ
������Խ��ߵľ���Ϊ4a�����ݹ��ɶ������ó������ı߳�Ϊ![]() nm�����������Ϊ��
nm�����������Ϊ��![]() ��3cm3��ÿ����������4��Bԭ�Ӻ�4��Nԭ�ӣ�����������Ϊ
��3cm3��ÿ����������4��Bԭ�Ӻ�4��Nԭ�ӣ�����������Ϊ![]() �������ܶȵĶ����У�b=
�������ܶȵĶ����У�b=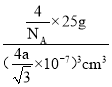 ���ó�NA=
���ó�NA=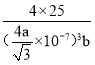 mol��1��
mol��1��
��Ϊ4�������壻��![]() ��
��![]() ��
��![]() ����
����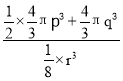 ��100%��
��100%��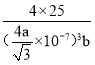 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ʵ����̲��ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ʵ��Ŀ�� | ʵ����� | |
A | ̽��ά����C�Ļ�ԭ�� | ��ʢ��2mL��ɫ�Ȼ�����Һ���Թ��еμ�Ũ��ά����C��Һ���۲���ɫ�仯 |
B | ����100mL1.0mol/L CuSO4��Һ | ��25.0gCuSO4��5H2O���100mL��Һ |
C | ��֤X��Һ���Ƿ���Fe2+ | ��X��Һ�еμӼ���������ˮ�����ټ�������KSCN��Һ���۲���Һ��ɫ�仯 |
D | ��ȥ����KNO3��������NaCl | ��������Ƴ��ȵı�����Һ����ȴ�ᾧ�����ˡ�ϴ�ӡ����� |
A.AB.BC.CD.D