ƒøƒ⁄»ð
°æƒø°øÔÿ”΢ıA◊‘™Àÿ–Œ≥…µƒªØ∫œŒÔ «÷ÿ“™µƒ∞εºÃÂ≤ƒ¡œ£¨”¶”√◊Óπ„∑∫µƒ «…ȪØÔÿ(GaAs)°£ªÿ¥œ¬¡–Œ £∫
£®1£©ª˘Ã¨Ga‘≠◊”µƒ∫ÀÕ‚µÁ◊”≈≈≤º ΩŒ™__________£¨ª˘Ã¨As‘≠◊”∫ÀÕ‚”–__________∏ˆŒ¥≥…∂‘µÁ◊”°£
£®2£©Ôÿ ß»•µÁ◊”µƒ÷º∂µÁ¿ÎƒÐ(µ•Œª£∫kJ°§mol-1)µƒ ˝÷µ“¿¥ŒŒ™577°¢1985°¢2962°¢6192£¨”…¥Àø…Õ∆÷™Ôÿµƒ÷˜“™ªØ∫œº€Œ™__________∫Õ+3°£…ȵƒµÁ∏∫–‘±»Ôÿ__________(ÃÓ°∞¥Û°±ªÚ°∞–°°±)°£
£®3£©±»Ωœœ¬¡–Ôÿµƒ¬±ªØŒÔµƒ»€µ„∫Õ∑–µ„£¨∑÷Œˆ∆‰±‰ªØπʬ…º∞‘≠“Ú£∫__________________________°£
Ôÿµƒ¬±ªØŒÔ | GaCl3 | GaBr3 | GaI3 |
»€µ„/°Ê | 77.75 | 122.3 | 211.5 |
∑–µ„/°Ê | 201.2 | 279 | 346 |
GaF3µƒ»€µ„≥¨π˝1000°Ê£¨ø…ƒÐµƒ‘≠“Ú «_______________________________________°£
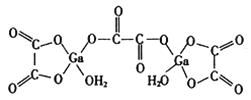
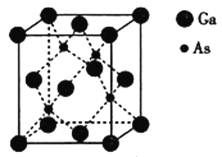
£®4£©∂˛ÀÆ∫œ≤ðÀ·ÔÿµƒΩ·ππ»ÁÕºÀ˘ 棨∆‰÷–Ôÿ‘≠◊”µƒ≈‰Œª ˝Œ™__________£¨≤ðÀ·∏˘÷–ú‘≠◊”µƒ‘”ªØ∑Ω ΩŒ™__________°£
£®5£©…ȪØÔÿ»€µ„Œ™1238°Ê£¨¡¢∑Ωæß∞˚Ω·ππ»ÁÕºÀ˘ 棨æß∞˚≤Œ ˝Œ™a=565pm£¨∏√æßõƒ¿ý–ÕŒ™__________£¨æßõƒ√Ð∂»Œ™__________(…ËNAŒ™∞¢∑¸º”µ¬¬Þ≥£ ˝µƒ ˝÷µ£¨¡–≥ˆÀ„ Ωº¥ø…)g°§cm-3°£
°æ¥∞∏°ø [ Ar ]3d104s24p1(ªÚ1s22s22p63s23p63d104s24p1) 3 +1(–¥+1°¢+2) ¥Û GaCl3°¢GaBr3°¢GaI3µƒ»€°¢∑–µ„“¿¥Œ…˝∏þ°£À¸√«æ˘Œ™∑÷◊”æßã¨Ω·ππœýÀ∆£¨œý∂‘∑÷◊”÷ ¡ø“¿¥Œ‘ˆ¥Û£¨∑÷◊”º‰◊˜”√¡¶“¿¥Œ‘ˆ«ø GaF3Œ™¿Î◊”æßà4 Sp2 ‘≠◊”æßà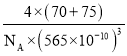
°æΩ‚Œˆ°ø(1) Gaµƒ‘≠◊”–Ú ˝Œ™31£¨À˘“‘ª˘Ã¨‘≠◊”µƒµÁ◊”≈≈≤º ΩŒ™[ Ar ]3d104s24p1(ªÚ1s22s22p6 3s23p6 3d104s24p1)£ªAsµƒ‘≠◊”–Ú ˝Œ™33£¨‘Úª˘Ã¨‘≠◊”µƒµÁ◊”≈≈≤º ΩŒ™[ Ar ]3d104s24p3£¨À˘“‘ª˘Ã¨As‘≠◊”∫ÀÕ‚”–3∏ˆŒ¥≥…∂‘µÁ◊”£ª
(2)µÁ¿ÎƒÐ «∆¯Ã¨‘≠◊” ß»•µÁ◊”À˘–Ë“™µƒƒÐ¡ø£¨”…Ôÿµƒ«∞Àƒº∂µÁ¿ÎƒÐø…÷™£¨∆‰÷˜“™ªØ∫œº€Œ™+1°¢+3£¨”…”⁄Asµƒ◊ÓÕ‚≤„µÁ◊”≈≈≤ºŒ™4s24p3£¨ «»´¬˙ªÚ∞ά˙£¨∂¯Gaµƒ◊ÓÕ‚≤„µÁ◊”≈≈≤ºŒ™4s24p1£¨Ãÿ± «4p1“◊ ßµÁ◊”£¨À˘“‘…ȵƒµÁ∏∫–‘±»Ôÿ¥Û£ª
(3)±Ì÷– ˝æ𜑠棨Ôÿµƒ¬±ªØŒÔµƒ»€µ„∫Õ∑–µ„∂º≤ª∏þ£¨«“∞¥’’¬»°¢‰Â°¢µ‚“¿¥Œ…˝∏þ£¨‘≠“Ú «À¸√«µƒ◊È≥…œýÕ¨£¨Ω·ππœýÀ∆£¨∂º «∑÷◊”æßã¨À˘“‘ÀÊ◊≈œý∂‘∑÷◊”÷ ¡øµƒ‘ˆ¥Û£¨∑÷◊”º‰◊˜”√¡¶‘ˆ¥Û£¨π »€∑–µ„…˝∏þ£ª∂¯GaF3µƒ»€µ„≥¨π˝1000°Ê£¨ «”…”⁄FµƒµÁ∏∫–‘∫Ð¥Û£¨–Œ≥…µƒGaF3 «¿Î◊”æßãª
(4)”…∂˛ÀÆ∫œ≤ðÀ·ÔÿµƒΩ·ππÕºø…µ√£¨Ôÿ‘≠◊”µƒ≈‰Œª ˝Œ™4£ª≤ðÀ·∏˘÷–ú‘≠◊””ÎÙ»ª˘÷–µƒÃº‘≠◊”µƒ‘”ªØ∑Ω ΩœýÕ¨£¨–Œ≥…µƒ∂º «∆Ω√ÊΩ·ππ£¨À˘“‘”¶∏√ «sp2‘”ªØ£ª
(5)”…”⁄∏√æßõƒ»€µ„∏þ£¨«“…È∫ÕÔÿ∂º≤ª «ªÓ∆√‘™Àÿ£¨À˘“‘∏√æßà«‘≠◊”æßã¨∆‰ªØ—ß ΩŒ™Ga4As4£¨∏√æß∞˚µƒ÷ ¡øm=![]() g£¨Ãª˝Œ™V =(565°¡10-10)3 cm3£¨‘Ú∆‰√Ð∂»Œ™
g£¨Ãª˝Œ™V =(565°¡10-10)3 cm3£¨‘Ú∆‰√Ð∂»Œ™![]() g/cm3°£
g/cm3°£

 ÷–øºΩ‚∂¡øºµ„æ´¡∑œµ¡–¥∞∏
÷–øºΩ‚∂¡øºµ„æ´¡∑œµ¡–¥∞∏ ∏˜µÿ∆⁄ƒ©∏¥œ∞Ãÿ—µæÌœµ¡–¥∞∏
∏˜µÿ∆⁄ƒ©∏¥œ∞Ãÿ—µæÌœµ¡–¥∞∏°æƒø°øCOS ∫ÕH2S «–Ì∂ý√∫ªØπ§≤˙∆∑µƒ‘≠¡œ∆¯°£“—÷™£∫
¢Ò.COS(g)+H2(g)![]() H2S(g)+CO(g) ¶§H=X kJ°§mol-1£ª
H2S(g)+CO(g) ¶§H=X kJ°§mol-1£ª
I.CO(g)+H2O(g)![]() CO2(g)+H2(g) ¶§H=-42 kJ°§mol-1£ª
CO2(g)+H2(g) ¶§H=-42 kJ°§mol-1£ª
£®1£©∂œ¡—1mol∑÷◊”÷–µƒªØ—ߺ¸À˘–ËŒ¸ ’µƒƒÐ¡ø»Áœ¬±ÌÀ˘ æ£∫
∑÷◊” | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
ƒÐ¡ø/kJ°§mol-1 | 1321 | 440 | 1076 | 680 | 930 | 1606 |
‘ÚX=_____________________°£
£®2£©œÚ10 L»ðª˝≤ª±‰µƒ√б’»ð∆˜÷–≥‰»Î1mol COS(g)°¢1mol H2(g)∫Õ1mol H2O(g)£¨Ω¯––…œ ˆ¡Ω∏ˆ∑¥”¶£¨‘⁄ƒ≥Œ¬∂»œ¬¥ÔµΩ∆Ω∫‚£¨¥À ±COµƒÃª˝∑÷ ˝Œ™4%£¨«“≤‚µ√¥À ±COSµƒŒÔ÷ µƒ¡øŒ™0.80mol£¨‘Ú∏√Œ¬∂»œ¬∑¥”¶Iµƒ∆Ω∫‚≥£ ˝Œ™_________________£®±£¡Ù¡ΩŒª”––ß ˝◊÷£©
£®3£©œ÷”–¡Ω∏ˆœýÕ¨µƒ2 L∫„»ð毻»£®”ÎÕ‚ΩÁ√ª”–»»¡øΩªªª£©√б’»ð∆˜M°¢N£¨‘⁄M ÷–≥‰»Î1mol CO∫Õ1molH2O£¨‘⁄N ÷–≥‰»Î1molCO2∫Õ1molH2£¨æ˘‘⁄700°Êœ¬ø™ º∞¥¢ÚΩ¯––∑¥”¶°£¥ÔµΩ∆Ω∫‚ ±£¨œ¬¡–Àµ∑®’˝»∑µƒ «_________°£
A.¡Ω»ð∆˜÷–CO µƒŒÔ÷ µƒ¡øM>N
B.¡Ω»ð∆˜÷–’˝∑¥”¶ÀŸ¬ M
C.»ð∆˜M ÷–COµƒ◊™ªØ¬ ”λð∆˜N ÷–CO2µƒ◊™ªØ¬ ÷Æ∫Õ–°”⁄1
D.¡Ω»ð∆˜÷–∑¥”¶µƒ∆Ω∫‚≥£ ˝M>N
£®4£©«‚¡ÚÀ·°¢ÃºÀ·æ˘Œ™∂˛‘™»ıÀ·£¨∆‰≥£Œ¬œ¬µƒµÁ¿Î≥£ ˝»Áœ¬±Ì£∫
H2CO3 | H2S | |
Ka1 | 4.4°¡ 10-7 | 1.3°¡10-7 |
Ka2 | 4.7°¡ 10-11 | 7.1°¡10-15 |
√∫µƒ∆¯ªØπ˝≥Ã÷–≤˙…˙µƒH2S ø…”√◊„¡øµƒNa2CO3»Ð“∫Œ¸ ’£¨∏√∑¥”¶µƒ¿Î◊”∑Ω≥Ã ΩŒ™______________£ª≥£Œ¬œ¬£¨”√100mL0.2mol°§L-1InaOH»Ð“∫Œ¸ ’448mL£®±Íøˆ£©H2S∆¯Ã£¨∑¥”¶∫ۻГ∫÷–¿Î◊”≈®∂»¥”¥ÛµΩ–°µƒÀ≥–ÚŒ™__________________________________°£
£®5£©25°Ê ±£¨”√Na2S≥¡µÌCu2+°¢Sn2+¡Ω÷÷Ω Ù¿Î◊” (M2+)£¨À˘–ËS2-◊ÓµÕ≈®∂»µƒ∂‘ ˝÷µ1gc(S2-
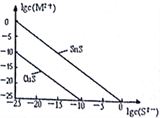
¢Ÿ25°Ê ±Ksp(CuS)=_______________°£
¢⁄25°Ê ±œÚ50mLµƒSn2+°¢Cu2+≈®∂»æ˘Œ™0.01mol/LµƒªÏ∫œ»Ð“∫÷–÷µŒº”»ÎNa2S»Ð“∫£¨µ±Na2S»Ð“∫º”µΩ150mL ±ø™ º…˙≥…SnS≥¡µÌ£¨‘Ú¥À ±»Ð“∫÷–Cu2+≈®∂»Œ™_____________mol/L°£
°æƒø°øƒ≥Õ¨—ß‘⁄—–æø‘™Àÿ–‘÷ µð±‰πʬ… µ—È ±£¨◊‘º∫…˺∆¡À“ªÃ◊ µ—È∑Ω∞∏£¨≤¢º«¬º¡À”–πÿ µ—Èœ÷œÛ(º˚œ¬±Ì£¨±Ì÷–µƒ°∞ µ—È∑Ω∞∏°±”ΰ∞ µ—Èœ÷œÛ°±«∞∫Û≤ª“ª∂® «∂‘”¶πÿœµ)°£
µ—È≤Ω÷Ë | µ—Èœ÷œÛ |
¢ŸΩ´√æÃı”√…∞÷Ω¥Úƒ•∫Û£¨∑≈»Î ‘πÐ÷–£¨º”»Î…Ÿ¡øÀÆ∫Û£¨º”»»÷¡ÀÆ∑–Ã⁄£ª‘ŸœÚ»Ð“∫÷–µŒº”∑”ٻГ∫ | A.∏°‘⁄ÀÆ√Ê…œ£¨»€≥…–°«Ú£¨Àƒ¥¶”Œ∂Ø£¨∑¢≥ˆ°∞ÀªÀª°±…˘£¨ÀÊ÷Æœ˚ ߣ¨»Ð“∫±‰≥…∫Ï…´ |
¢⁄œÚ–¬÷∆µƒNa2S»Ð“∫÷–µŒº”–¬÷∆µƒ¬»ÀÆ | B.”–∆¯ÃÂ≤˙…˙£¨»Ð“∫±‰≥…«≥∫Ï…´ |
¢€Ω´“ª–°øÈΩ Ùƒ∆∑≈»ÎµŒ”–∑”ٻГ∫µƒ¿‰ÀÆ÷– | C.æÁ¡“∑¥”¶£¨—∏ÀŸ≤˙…˙¥Û¡øŒÞ…´∆¯Ã |
¢ÐΩ´√æÃıÕ∂»Îœ°—ŒÀ·÷– | D.∑¥”¶≤ª Æ∑÷æÁ¡“£¨≤˙…˙ŒÞ…´∆¯Ã |
¢ðΩ´¬¡ÃıÕ∂»Îœ°—ŒÀ·÷– | E.…˙≥…∞◊…´Ω∫◊¥≥¡µÌ£¨ºÃ∂¯≥¡µÌœ˚ ß |
¢ÞœÚAlCl3»Ð“∫÷–µŒº”NaOH»Ð“∫÷¡π˝¡ø | F.…˙≥…µ≠ª∆…´≥¡µÌ |
«Îƒ„∞Ô÷˙∏√Õ¨—ß’˚¿Ì≤¢ÕÍ≥… µ—ȱ®∏Ê°£
(1) µ—ȃøµƒ£∫—–æø__________________‘™Àÿ–‘÷ µð±‰πʬ…°£
(2) µ—È”√∆∑£∫ ‘º¡£∫Ω Ùƒ∆°¢√æÃı°¢¬¡Ãı°¢œ°—ŒÀ·°¢–¬÷∆¬»ÀÆ°¢–¬÷∆Na2S»Ð“∫°¢AlCl3»Ð“∫°¢NaOH»Ð“∫°¢∑”ٻГ∫µ»°£
“«∆˜£∫______°¢______°¢¬À÷Ω°¢ ‘πк–°¢Ω∫Õ∑µŒπа¢ƒ˜◊”°¢–°µ∂°¢≤£¡ß∆¨°¢…∞÷Ω°¢ª≤Òµ»°£
(3) µ—ȃ⁄»ð£∫(ÃÓ–¥”Î µ—È≤Ω÷Ë∂‘”¶µƒ µ—Èœ÷œÛµƒ±ý∫≈∫Õ¢Ÿ¢⁄µƒªØ—ß∑Ω≥Ã Ωº∞¥À µ—ȵƒΩ·¬€)
µ—ȃ⁄»ð | ¢Ÿ | ¢⁄ | ¢€ | ¢Ð | ¢ð | ¢Þ |
µ—Èœ÷œÛ(ÃÓA°´F) | __ | __ | __ | __ | __ | __ |
¢Ÿ__________________________________________________£ª
¢⁄___________________________________________________£ª
¥À µ—ȵƒΩ·¬€£∫__________________________________________________°£
°æƒø°ø”–¢Ò°¢¢Ú°¢¢Û3∏ˆÃª˝æ˘Œ™0.5Lµƒ∫„»ð√б’»ð∆˜£¨‘⁄¢Ò°¢¢Ú°¢¢Û÷–∞¥≤ªÕ¨Õ∂¡œ±»(Z)≥‰»ÎHCl∫ÕO2(»Áœ¬±Ì)£¨º”»Î¥þªØº¡∑¢…˙∑¥”¶£∫4HCl(g)+O2(g)![]() 2Cl2(g)+2H2O(g)°˜H°£HClµƒ∆Ω∫‚◊™ªØ¬ ”ÎZ∫ÕTµƒπÿœµ»ÁÕºÀ˘ æ°£
2Cl2(g)+2H2O(g)°˜H°£HClµƒ∆Ω∫‚◊™ªØ¬ ”ÎZ∫ÕTµƒπÿœµ»ÁÕºÀ˘ æ°£
»ð∆˜ | ∆ º ± | ||
T/°Ê | n(HCl)/mol | Z | |
¢Ò | 300 | 0.25 | a |
¢Ú | 300 | 0.25 | b |
¢Û | 300 | 0.25 | 4 |
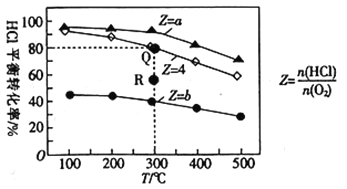
œ¬¡–Àµ∑®≤ª’˝»∑µƒ «£® £©
A. °˜H<0
B. a<4<b
C. »Ù»ð∆˜¢Û∑¥”¶ƒ≥ ±øÃ¥¶”⁄Rµ„£¨‘ÚRµ„µƒ∑¥”¶ÀŸ¬ £∫v(’˝)>v(ƒÊ)
D. 300°Ê ±£¨∏√∑¥”¶∆Ω∫‚≥£ ˝µƒ÷µŒ™320