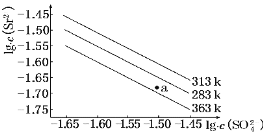
��Ŀ����
����Ŀ�����������£����и�����������Ӧ�������¿��ܴ���������ǣ�������
A.��ʹpH��ֽ������Һ�У�CO32-��K����Cl����Na��
B.��ˮ���������c(OH��)��1��10��10 mol��L��1����Һ�У�NO3-��Mg2����Na����SO42-
C.��c(OH��)/c(H��)��1��1012����Һ�У�NH4+��Fe3����Cl����NO3-
D.![]() ��10��10 mol��L��1����Һ�У�Na����HCO3-��Cl����K��
��10��10 mol��L��1����Һ�У�Na����HCO3-��Cl����K��
���𰸡�B
��������
A����Һ��ʹpH��ֽ��죬˵����Һ�к��д���H+����Һ��CO32-��H+��Ӧ�����ܴ������棬��A���������⣻
B�������£���ˮ���������c(OH��)��1��10��10 mol��L��1<1��10-7 mol��L��1��ˮ�ĵ����ܵ����ƣ���ҺΪ���Ի���ԣ�����ҺΪ����ʱ��NO3-��Mg2����Na����SO42-�ܴ������棬����ҺΪ����ʱ��Mg2����OH-��Ӧ����������Mg(OH)2���ʸ������£���Һ�������ܴ������棬��B�������⣻
C����c(OH��)/c(H��)��1��1012��Kw=1��10-14��֪����Һ��c(H+)=1��10-13��c(OH-)=0.1mol/L����Һ��NH4+��Fe3������OH-��Ӧ����������ʣ��ʸ���Һ�����Ӳ��ܴ������棬��C���������⣻
D����![]() ��c(OH-)=10��10 mol��L��1��Kw=1��10-14��֪����Һ�����ԣ�HCO3-����H+��Ӧ����Һ�����Ӳ��ܴ������棬��D���������⡣
��c(OH-)=10��10 mol��L��1��Kw=1��10-14��֪����Һ�����ԣ�HCO3-����H+��Ӧ����Һ�����Ӳ��ܴ������棬��D���������⡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���������ȣ�ClO2���㷺Ӧ����ֽ��Ư�ס�ɱ��������ˮ��������������ҵ�����ü״���ԭNaClO3�ķ����Ʊ�ClO2�������������£�
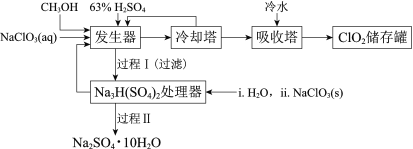
��֪��a�����������Ʊ�ClO2�ķ�Ӧ��12NaClO3+8H2SO4+3CH3OH= 12ClO2��+3HCOOH+4Na3H(SO4)2��+9H2O
b��������ʵ��۷е㣺
���� | CH3OH | HCOOH | ClO2 |
�۵�/�� | ��97 | 9 | ��59 |
�е�/�� | 65 | 101 | 11 |
(1)ClO2������ֽ��Ư�ס�ɱ���������������______�ԡ�
(2)��ȴ�����ڷ���ClO2������CH3OH��Ӧ���Ƶ�����¶�Ϊ______������ĸ����
A��0~10�� B��20~30�� C��60~70��
(3)�����̢���̢���Ի��â����Na2SO4��10H2O����ʹ����ԭ��ѭ�����á�
��֪��Na2SO4��10H2O��Na2SO4���ܽ����������ͼ��
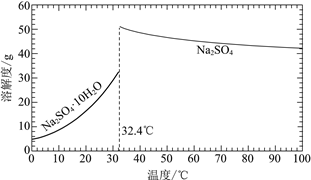
��Na3H(SO4)2�������л��â��ʱ�����NaClO3���壬��â���ܽ�ƽ��ĽǶȽ�����ԭ��______��
�ڽ��Na2SO4��10H2O��Na2SO4���ܽ�����ߣ����̢�IJ����ǣ���32.4�����������______��
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ����NaClO3��______��