��Ŀ����
����Ŀ���������ȣ�ClO2���㷺Ӧ����ֽ��Ư�ס�ɱ��������ˮ��������������ҵ�����ü״���ԭNaClO3�ķ����Ʊ�ClO2�������������£�
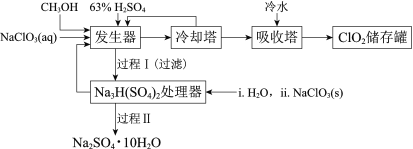
��֪��a�����������Ʊ�ClO2�ķ�Ӧ��12NaClO3+8H2SO4+3CH3OH= 12ClO2��+3HCOOH+4Na3H(SO4)2��+9H2O
b��������ʵ��۷е㣺
���� | CH3OH | HCOOH | ClO2 |
�۵�/�� | ��97 | 9 | ��59 |
�е�/�� | 65 | 101 | 11 |
(1)ClO2������ֽ��Ư�ס�ɱ���������������______�ԡ�
(2)��ȴ�����ڷ���ClO2������CH3OH��Ӧ���Ƶ�����¶�Ϊ______������ĸ����
A��0~10�� B��20~30�� C��60~70��
(3)�����̢���̢���Ի��â����Na2SO4��10H2O����ʹ����ԭ��ѭ�����á�
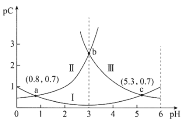
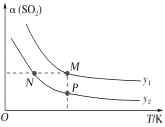
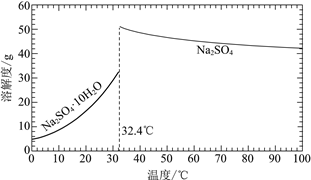
��֪��Na2SO4��10H2O��Na2SO4���ܽ����������ͼ��
��Na3H(SO4)2�������л��â��ʱ�����NaClO3���壬��â���ܽ�ƽ��ĽǶȽ�����ԭ��______��
�ڽ��Na2SO4��10H2O��Na2SO4���ܽ�����ߣ����̢�IJ����ǣ���32.4�����������______��
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ����NaClO3��______��
���𰸡����� B ![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O������ ��ȴ�ᾧ�����ˣ�ϴ�ӣ����� H2SO4
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O������ ��ȴ�ᾧ�����ˣ�ϴ�ӣ����� H2SO4
��������
�����̿�֪�����������Ʊ�ClO2, ��ȴ�����ڷ���ClO2������CH3OH����������������ˮ����ClO2������ٴ��棻�����������ɵ�Na3H(SO4)2����Na3H(SO4)2����������H2O2��NaClO3�����õ�Na2SO4��10H2O��
��1��ClO2������ֽ��Ư�ס�ɱ��������������������ԣ��ʴ�Ϊ��������
��2����ȴ�����ڷ���ClO2������CH3OH�����ݱ�����������ʵ��ܽ�ȣ����Ƶ��¶�Ӧ��ʹCH3OHҺ��������ClO2����Һ����ֻ��B����ʣ���ѡB��
��3�����ڴ������лᷢ����Ӧ��![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O���������ʴ�Ϊ��
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O���������ʴ�Ϊ��![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O��������
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O��������
�ڹ��̢�IJ���Ϊ��32.4���������������ȴ�ᾧ�����ˣ�ϴ�ӣ�����ʴ�Ϊ����ȴ�ᾧ�����ˣ�ϴ�ӣ����
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ��ΪNaClO3��H2SO4,�ʴ�Ϊ��H2SO4��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijͬѧ��̽��FeCl3��Һ��NaHSO3��Һ�ķ�Ӧ����ơ����ʵ�鲢��¼���£�
װ�� | ��Ӧʱ�� | ʵ������ |
| 0~1 min | �������ɫ����Fe(OH)3���д̼�����ζ����SO2�ݳ� |
1~30 min | ����Ѹ���ܽ��γɺ�ɫ��Һ�������Һ��Ϊ��ɫ��֮����ɫ | |
30 min�� | ������Ӵ����ֵ��ϲ���Һ�ֱ�Ϊdz��ɫ�������Ϊdz��ɫ |
��֪�� Fe(OH)3+SO32-![]()
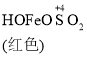
![]()
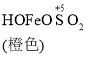
![]() Fe2++SO42-
Fe2++SO42-
�����йط�Ӧ���ʺͻ�ѧƽ��˵������ȷ����
A. ���ɺ�ɫ�����ķ�Ӧ���ʽϿ죬��ɫ��������ɳ�ɫ���������ʽ���
B. ������Һ��SO32-Ũ�ȿ��Լ��ٺ��ɫ����Fe(OH)3Ѹ���ܽ�
C. O2�������£���ɫHOFeOSO2Ũ���½���ƽ��![]() ���������ƶ�
���������ƶ�
D. 30minʱ���������ɫ��Һ�еμ�����FeCl3��Һ������������
����Ŀ�����ʡ�ī��ֽ���������й���ͳ�Ļ��б���Ϊ���ķ��ı���������˵���д�����ǣ� ��
A | B | C | D |
|
|
|
|
�����շ��ɼ������������������α | ī����Ҫ�ɷ���̼���� | ֽ����Ҫ�ɷ����ںϳɲ��� | ��ʯ��������̨�Ĺ����������仯 |
A.AB.BC.CD.D