��Ŀ����
��15�֣�X��Y��Z��W���������������ת����ϵ������X��W���ʣ�Y��ZΪ������,δ�г���Ӧ��������

����Z�������г��õĵ�ζƷ��W��������Һ��������
��1�������£�X����ɫ�� ��
��2����ҵ��Z�ж�����;���û�ѧ����ʽ��ʾZ��һ����; ��
��3�����������õ�Z�����˵���أ�����X��Y��Һ��Ӧʱ���Եõ�һ�ֵ����Σ��˷�Ӧ�����ӷ���ʽ�� ��
��������ij��ѧ����С���о�ŨH2SO4�������ԵĽ��۲�������ʵ����֤��
��һ����ΪH2SO4Ũ�ȴ���60%���;���һ����ǿ�����ԣ�ԽŨ������Խǿ��60%���µ�H2SO4��Ҫ���ֵ������ԣ������ϲ�����ǿ�����ԡ�
���¶�ҲӰ�������ԣ���ͭ�������ŨH2SO4�з�Ӧ�����ԣ�������ȾͿ��Թ۲쵽��������
��98%��ŨH2SO4���ʵ���Ũ��Ϊ18.4 mol/L,�ܶ�Ϊ1.84 g��cm-3������������Ϣ�ش�?
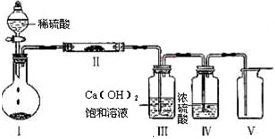
��1����ͼװ�ã�ͬѧ�������֧�ܵ��Թ��з���ͭ�ۺ�3 mLˮ��Ȼ��ӷ�Һ©���м�98%��ŨH2SO4 0.5 mL�����������ڣ����Թ���Һ��û�����Ա仯���������ݽ���
ԭ�� ��

��2����ͬѧ������ʾװ��ֱ�Ӵӷ�Һ©�����ټ�ŨH2SO4 10 mL��ͭƬ��Ӧ��������Թ������۲쵽��ײ�ͬ����������к�ɫ���ʳ����⣬����
�� ��
ԭ���� ��
�� ��
ԭ���� ��
��1������ɫ ��1�֣�
��2��2 NaCl +2 H2O H2��+ Cl2�� + 2
NaOH ��2�֣��𰸺��������֣�
H2��+ Cl2�� + 2
NaOH ��2�֣��𰸺��������֣�
��3��3 Cl2 + I�� + 3 H2O == 6 Cl- + IO3�� + 6 H+��2�֣�
��
��1��H2SO4��Һ����������Ϊ23%<60%���������Բ�ǿ����������������2�֣�
��2����Һ���dz��ɫ����2�֣���Һ�к���Cu2+ ����2�֣�
���а�ɫ�������Թܵײ���������2�֣�
98%ŨH2SO4����ˮ�ԣ�������ˮCuSO4����2�֣�
��������






 X��Y��Z��W���ֻ�������ɳ���Ԫ����ɣ�����X��������Ԫ�أ�Y��Z��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ����������ͼת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ������ش�
X��Y��Z��W���ֻ�������ɳ���Ԫ����ɣ�����X��������Ԫ�أ�Y��Z��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ����������ͼת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ������ش�