��Ŀ����
 X��Y��Z��W���ֻ�������ɳ���Ԫ����ɣ�����X��������Ԫ�أ�Y��Z��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ����������ͼת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ������ش�
X��Y��Z��W���ֻ�������ɳ���Ԫ����ɣ�����X��������Ԫ�أ�Y��Z��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���壮�����ֻ����������ͼת����ϵ�����ַ�Ӧ����P��Ӧ��������ȥ������ش���1��W�Ļ�ѧʽ��
��2��X��Y����Һ�з�Ӧ�����ӷ���ʽ��
��3����Y��Һ��ͨ�����������Ƶ�ij�������������г��õ�Ư�ס����������ʣ��÷�Ӧ�Ļ�ѧ����ʽ��
��4��X���е�����Ԫ��֮�䣨���֡����ֻ����֣�����ɶ��ֻ����ѡ������ijЩ���������ͼ��װ�ã��г̶ֹ�װ������ȥ���Ʊ����ռ����������R���壬��֪R�����X������Ԫ���е�һ����ɣ�װ�â��в�����ɫ��������ش��������⣺
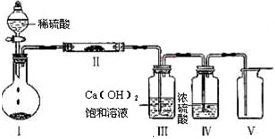
����װ�â��еĹ���ΪZ����װ�â������ʵĻ�ѧʽ��
������X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������R���壬��û�����Ļ�ѧʽ��
��������1��X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��˵�����߾�Ϊ��Ԫ�صĻ����X��������Ԫ�أ��ڼ���������������Z�������ֻ������Ϊ������Ԫ����ɣ���WΪ��ɫ��ζ�����壬���Ƴ�XΪNaHCO3��YΪNaOH��ZΪNa2CO3��WΪCO2��
��2������������̼�����Ʒ�Ӧ����̼������ˮ��
��3���������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ��
��4����X����������Ԫ��Ϊ��Na��H��C��O������Ԫ��֮����ɵĻ�����Ʊ����ռ������������ɫ����R��R�����X������Ԫ���е�һ����ɣ�Ϊ���嵥�ʣ�װ�â��в�����ɫ��������װ�â��з�Ӧ����CO2��ZΪNaHCO3��Na2CO3���壬��װ�â��з�Ӧ�������嵥��R��ӦΪ������̼��������Ʒ�Ӧ����������װ�â��ȥ�����л��еĶ�����̼��װ�â�Ϊ����������װ�â����ռ���������O2��
��װ�â����ռ���������O2��O2����ȡҲ������H2O2 �ڶ������������������·ֽ��Ƶã��ݴ˽��
��2������������̼�����Ʒ�Ӧ����̼������ˮ��
��3���������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ��
��4����X����������Ԫ��Ϊ��Na��H��C��O������Ԫ��֮����ɵĻ�����Ʊ����ռ������������ɫ����R��R�����X������Ԫ���е�һ����ɣ�Ϊ���嵥�ʣ�װ�â��в�����ɫ��������װ�â��з�Ӧ����CO2��ZΪNaHCO3��Na2CO3���壬��װ�â��з�Ӧ�������嵥��R��ӦΪ������̼��������Ʒ�Ӧ����������װ�â��ȥ�����л��еĶ�����̼��װ�â�Ϊ����������װ�â����ռ���������O2��
��װ�â����ռ���������O2��O2����ȡҲ������H2O2 �ڶ������������������·ֽ��Ƶã��ݴ˽��
����⣺X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��˵�����߾�Ϊ��Ԫ�صĻ����X��������Ԫ�أ��ڼ���������������Z�������ֻ������Ϊ������Ԫ����ɣ���WΪ��ɫ��ζ�����壬���Ƴ�XΪNaHCO3��YΪNaOH��ZΪNa2CO3��WΪCO2��
��1�������Ϸ�����֪WΪCO2���ʴ�Ϊ��CO2��
��2������������̼�����Ʒ�Ӧ����̼������ˮ����Ӧ���ӷ���ʽΪ��HCO3-+OH-=CO32-+H2O��
�ʴ�Ϊ��HCO3-+OH-=CO32-+H2O��
��3���������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ����Ӧ����ʽΪ��2NaOH+C12=NaClO+NaCl+H2O��
�ʴ�Ϊ��2NaOH+C12=NaClO+NaCl+H2O��
��4����X����������Ԫ��Ϊ��Na��H��C��O������Ԫ��֮����ɵĻ�����Ʊ����ռ������������ɫ����R��R�����X������Ԫ���е�һ����ɣ�Ϊ���嵥�ʣ�װ�â��в�����ɫ��������װ�â��з�Ӧ����CO2��ZΪNaHCO3��Na2CO3���壬��װ�â��з�Ӧ�������嵥��R��ӦΪ������̼��������Ʒ�Ӧ����������װ�â��ȥ�����л��еĶ�����̼��װ�â�Ϊ����������װ�â����ռ���������O2��
�ʴ�Ϊ��Na2O2����ȥ�����л��еĶ�����̼������������
��װ�â����ռ���������O2��O2����ȡҲ������2H2O2
2H2O+O2�����У���������װ���Ǣ���������װ�â�ȥ������Һ©����ʢ��H2O2��Բ����ƿ��ʢ�Ŷ������̣�
�ʴ�Ϊ��H2O2����װ�â�ȥ������Һ©����ʢ��H2O2��Բ����ƿ��ʢ�Ŷ������̣�
��1�������Ϸ�����֪WΪCO2���ʴ�Ϊ��CO2��
��2������������̼�����Ʒ�Ӧ����̼������ˮ����Ӧ���ӷ���ʽΪ��HCO3-+OH-=CO32-+H2O��
�ʴ�Ϊ��HCO3-+OH-=CO32-+H2O��
��3���������������Ʒ�Ӧ�����Ȼ��ơ�����������ˮ����Ӧ����ʽΪ��2NaOH+C12=NaClO+NaCl+H2O��
�ʴ�Ϊ��2NaOH+C12=NaClO+NaCl+H2O��
��4����X����������Ԫ��Ϊ��Na��H��C��O������Ԫ��֮����ɵĻ�����Ʊ����ռ������������ɫ����R��R�����X������Ԫ���е�һ����ɣ�Ϊ���嵥�ʣ�װ�â��в�����ɫ��������װ�â��з�Ӧ����CO2��ZΪNaHCO3��Na2CO3���壬��װ�â��з�Ӧ�������嵥��R��ӦΪ������̼��������Ʒ�Ӧ����������װ�â��ȥ�����л��еĶ�����̼��װ�â�Ϊ����������װ�â����ռ���������O2��
�ʴ�Ϊ��Na2O2����ȥ�����л��еĶ�����̼������������
��װ�â����ռ���������O2��O2����ȡҲ������2H2O2
| ||
�ʴ�Ϊ��H2O2����װ�â�ȥ������Һ©����ʢ��H2O2��Բ����ƿ��ʢ�Ŷ������̣�
���������⿼���Ϊ�ۺϣ��漰������ƶϡ�������Ʊ��ȣ���Ŀ�Ѷ��еȣ�ע��̼���ơ�̼�����Ƶ����ʵ����ʣ����ճ���������Ʊ�������

��ϰ��ϵ�д�
�����Ŀ
 ��2011?���գ�ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�����������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29��
��2011?���գ�ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�����������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29��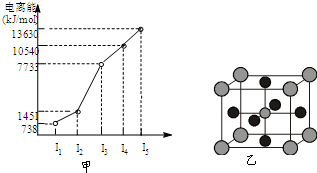 ��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn��
��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn�� ԭ������С��36��X��Y��Z��W����Ԫ�أ�����Xԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵ��ӣ�Yԭ�ӻ�̬ʱ���������������ڲ��������3����ZԪ�ص�����������ˮ�����������ǿ��W��ԭ������Ϊ30��
ԭ������С��36��X��Y��Z��W����Ԫ�أ�����Xԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵ��ӣ�Yԭ�ӻ�̬ʱ���������������ڲ��������3����ZԪ�ص�����������ˮ�����������ǿ��W��ԭ������Ϊ30��