��Ŀ����
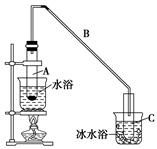
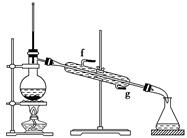
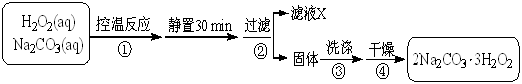
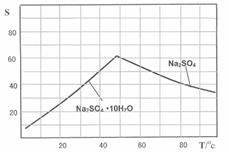
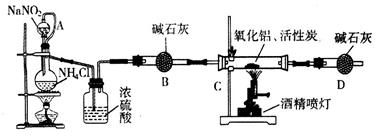
����ǰ��һ��ʳ�������㸡�����Ϻ�����°��������������ȴ������㸡�顱�Ļ�ѧ�ɷ�ʵΪ��̼���ƣ�ʹ�ò������������ˣ���̼���Ʊ��������������岻����Σ������̼���ƣ��׳ƹ���˫��ˮ����ѧʽΪ2Na2CO3��3H2O2����һ�����Σ��ǰ�ɫ����״��ĩ�����Էֽ�Ϊ̼���ƺ������⡣ij̽��С���Ʊ���̼���Ʋ��ⶨ��Ʒ��H2O2�ĺ��������Ʊ����̺�װ��ʾ��ͼ���£�
��֪��50 ��Cʱ 2Na2CO3��3H2O2 (s) ��ʼ�ֽ�
����Ӧ 2Na2CO3 (aq) + 3H2O2 (aq) 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0
����Ӧ 2H2O2 = 2H2O + O2��
�ζ���Ӧ 6KMnO4 + 5(2Na2CO3��3H2O2) +19H2SO4 = 3K2SO4 + 6MnSO4+10Na2SO4 + 10CO2 �� + 15O2�� + 34H2O

����������Ϣ�ش��������⣺
��1���Ʋ�ͼ��֧�ܵ����ÿ����� ��
��2������ٵĹؼ��ǿ����¶ȣ����װ��ͼ�������ʩ�� ��
�� ��
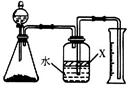
��3������ҺX�м�������NaCl�������ˮ�Ҵ�����������̼���ƣ�ԭ���� ��
��4���������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ���� ��
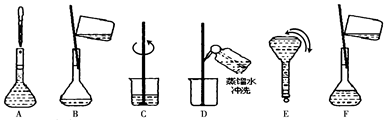
��5���ⶨ��Ʒ��H2O2�����������ķ����ǣ�ȷ��ȡ0.2000g��̼������Ʒ��250 mL ��ƿ�У���50 mL ����ˮ�ܽ⣬�ټ�50 mL 2.000 mol��L��1 H2SO4 (H2SO4����)����0.002000mol��L��1 KMnO4����Һ�ζ����յ�ʱ����30.00 mL��
�ٵζ�ǰ���ζ�������KMnO4����Һ��ϴ2��3�Σ���ϴ�IJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4�� ��
��������Ʒ��H2O2�����������������ʽΪ ��ֻ�г���ʽ�������κ����㣡H2O2��ʽ��Ϊ34.00����

��֪��50 ��Cʱ 2Na2CO3��3H2O2 (s) ��ʼ�ֽ�
����Ӧ 2Na2CO3 (aq) + 3H2O2 (aq)
 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0����Ӧ 2H2O2 = 2H2O + O2��
�ζ���Ӧ 6KMnO4 + 5(2Na2CO3��3H2O2) +19H2SO4 = 3K2SO4 + 6MnSO4+10Na2SO4 + 10CO2 �� + 15O2�� + 34H2O

����������Ϣ�ش��������⣺
��1���Ʋ�ͼ��֧�ܵ����ÿ����� ��
��2������ٵĹؼ��ǿ����¶ȣ����װ��ͼ�������ʩ�� ��
�� ��
��3������ҺX�м�������NaCl�������ˮ�Ҵ�����������̼���ƣ�ԭ���� ��
��4���������ѡ����ˮ�Ҵ�ϴ�Ӳ�Ʒ��Ŀ���� ��
��5���ⶨ��Ʒ��H2O2�����������ķ����ǣ�ȷ��ȡ0.2000g��̼������Ʒ��250 mL ��ƿ�У���50 mL ����ˮ�ܽ⣬�ټ�50 mL 2.000 mol��L��1 H2SO4 (H2SO4����)����0.002000mol��L��1 KMnO4����Һ�ζ����յ�ʱ����30.00 mL��
�ٵζ�ǰ���ζ�������KMnO4����Һ��ϴ2��3�Σ���ϴ�IJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4�� ��
��������Ʒ��H2O2�����������������ʽΪ ��ֻ�г���ʽ�������κ����㣡H2O2��ʽ��Ϊ34.00����
��17�֣�
��1��ƽ��ѹǿ��������Һ˳�����£�2�֣���ƽ��ѹǿ����ѹ����2�֣�����������������֣�
��2����ˮԡ �������� ��ͨ����Һ©��������������Σ��μ�H2O2��Һ����6�֣���2�֡�˵�������������Ŀ����ʹ��Ӧ��������������ɢȥ��
��3�����Ͳ�Ʒ�����̼���ƣ����ܽ��ԣ��ȣ���2�֣���˼�������֣�
��4��ϴȥˮ�ݣ����ڸ��[1��]�����ٹ������ܽ����ʧ[1��]����2�֣�����������������֣���
��5������бת���ζ�����ϴ�����ζ����ڱڣ���1�֣�Ȼ���������ϴҺ���¶˷ų� (1��)[��2�֣�����Ҫ�㣬һ����бת�������Ǵ�������ϴҺ���¶˷ų���û�д�Ҫ�㲻���֡�]
�� ��3�֣�
��3�֣�
[���չ㶫�߿��������������ʽ��ȫ��ȷ��û����λ��100%���۷֡��������������0�֣�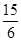 ��2.5���治�۷֣�����������������������2��]
��2.5���治�۷֣�����������������������2��]
��1��ƽ��ѹǿ��������Һ˳�����£�2�֣���ƽ��ѹǿ����ѹ����2�֣�����������������֣�
��2����ˮԡ �������� ��ͨ����Һ©��������������Σ��μ�H2O2��Һ����6�֣���2�֡�˵�������������Ŀ����ʹ��Ӧ��������������ɢȥ��
��3�����Ͳ�Ʒ�����̼���ƣ����ܽ��ԣ��ȣ���2�֣���˼�������֣�
��4��ϴȥˮ�ݣ����ڸ��[1��]�����ٹ������ܽ����ʧ[1��]����2�֣�����������������֣���
��5������бת���ζ�����ϴ�����ζ����ڱڣ���1�֣�Ȼ���������ϴҺ���¶˷ų� (1��)[��2�֣�����Ҫ�㣬һ����бת�������Ǵ�������ϴҺ���¶˷ų���û�д�Ҫ�㲻���֡�]
��
 ��3�֣�
��3�֣�[���չ㶫�߿��������������ʽ��ȫ��ȷ��û����λ��100%���۷֡��������������0�֣�
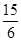 ��2.5���治�۷֣�����������������������2��]
��2.5���治�۷֣�����������������������2��]�����������1����ͼ���ɺ�ѹ��Һ©���ƶϣ�֧�ܵ�������ƽ��ѹǿ��������Һ˳�����£���2�������⣬����Ӧ�Ƿ��ȷ�Ӧ���¶ȹ��ߵ���H2O2�ֽ⣬��������¶ȣ�����H2O2�ķֽ⣬ͼ������ˮԡ���������������ܷ�ֹ��ӦҺ�¶ȹ��ߣ������¶ȸߵͿ��Ƶ���˫��ˮ���ٶȣ�Ҳ�ܿ��Ʒ�ӦҺ���¶ȣ���3������������Һ�ǹ�̼���Ƶı�����Һ����������NaCl����ˮ�Ҵ������ܽ������ܽ�ȣ�������Һ��������̼���ƹ��壻��4����ˮ�Ҵ�ϴ�ӵ�Ŀ���dz�ȥ�����л��е�ˮ�֣������ڹ���ĸ��ͬʱ���ܼ��ٹ�̼���Ƶ��ܽ⣬������ʧ����5������ϴ��ʽ�ζ��ܵIJ��������ǣ��ر���ʽ�ζ��ܻ�������ζ�����ע������KMnO4����Һ����бת���ζ�����ϴ�����ζ����ڱڣ�Ȼ���������ϴҺ���¶˷ų�������n=c?V����ζ�ʱ������0.002000��30.00��10��3mol KMnO4���ζ���ӦΪ6KMnO4+5(2Na2CO3��3H2O2)+19H2SO4=3K2SO4+6MnSO4+10Na2SO4+10CO2��+15O2��+ 34H2O�����ݸ����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ�������������Ĺ�̼���ƣ�2Na2CO3��3H2O2��Ϊ0.002000��30.00��10��3��5/6mol������2Na2CO3��3H2O2=2Na2CO3+3H2O2�����ݸ����ʵ�ϵ��֮�ȵ������ʵ���֮�ȣ�����Ʒ��H2O2�����ʵ���Ϊ0.002000��30.00��10��3��5/6��3mol������m=n?M������Ʒ��H2O2������Ϊ0.002000��30.00��10��3��5/6��3��34.00g��������Ʒ����Ϊ0.2000g������Ʒ��H2O2�����������������ʽΪ0.002000��30.00��10��3��5/6��3��34.00/0.2000��100%��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 ��
��