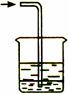
��Ŀ����
��2.4 mol/L��H2SO4��Һ����100mLŨ��Ϊ0.2 mol/L��ϡH2SO4���ش��������⣺
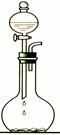
��1��������Ͳ��ȡ2.4 mol/L��H2SO4��Һ������� mL��
��2����Һ���Ƶ�����Ļ����������£�
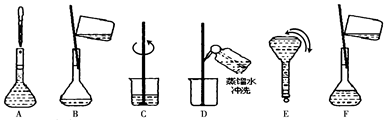
������ʵ�鲽��A��F��ʵ������Ⱥ��������___________________ ��
��3������ʵ�鲽��A��B��E��F���õ�����������Ϊ__________��
��4��ȡ����������Һ10mL������BaCl2��Һ��Ӧ�����ɰ�ɫ����0.48 g�������Һ
Ũ�� 0.2 mol/L������ڡ������ڡ���С�ڡ���,��ɴ����IJ�������
�� ��
a. ����ʱ��������ƿ�� b. ����Ͳȡ2.4 mol/LH2SO4��Һʱ���Ӷ�����
c. ����ƿʹ��ǰδ���d. ʹ�õ��ձ��Ͳ�����δϴ�ӳ��ף�
e. ����ʱ������ˮ��������ƿ����

��1��������Ͳ��ȡ2.4 mol/L��H2SO4��Һ������� mL��
��2����Һ���Ƶ�����Ļ����������£�
������ʵ�鲽��A��F��ʵ������Ⱥ��������___________________ ��
��3������ʵ�鲽��A��B��E��F���õ�����������Ϊ__________��
��4��ȡ����������Һ10mL������BaCl2��Һ��Ӧ�����ɰ�ɫ����0.48 g�������Һ
Ũ�� 0.2 mol/L������ڡ������ڡ���С�ڡ���,��ɴ����IJ�������
�� ��
a. ����ʱ��������ƿ�� b. ����Ͳȡ2.4 mol/LH2SO4��Һʱ���Ӷ�����
c. ����ƿʹ��ǰδ���d. ʹ�õ��ձ��Ͳ�����δϴ�ӳ��ף�
e. ����ʱ������ˮ��������ƿ����
��8�֣���1��8.3��2�֣�
��2��CBDFAE����2�֣�
��3��100mL����ƿ����1�֣�
��4�����ڣ�1�֣� a��2�֣�
��2��CBDFAE����2�֣�
��3��100mL����ƿ����1�֣�
��4�����ڣ�1�֣� a��2�֣�
�����������1������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬��Ũ��������ΪxmL������xmL��2.4mol/L=100mL��0.2mol/L����ã�x��8.3������Ӧ��ȡ�����������8.3mL���ʴ�Ϊ��8.3mL��
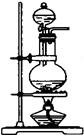
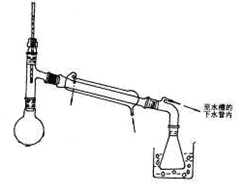
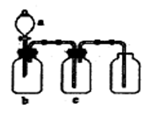
��2�����Ʋ�������ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������10mL��Ͳ��ȡ���õ���ͷ�ιܣ����ᣬ���ձ���ϡ�ͣ����ò��������裬��ȴ��ת�Ƶ�100mL����ƿ�У����ò�����������ϴ���ձ��벣����2��3�Σ�����������ƿ�ڣ�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ��Ǻ�ƿ���������ߵ�ҡ�ȣ��ʲ���˳��ΪCBDFAE��
��3����ͼ�й۲�õ���A��B��E��F���õ�����������Ϊ100mL����ƿ��
��4����ɫ���������ᱵ�����ᱵ�����ʵ���Ϊ0.48g��233g/mol="0.48/233" mol������n��H2SO4��=n��BaSO4��="=0.48/233" mol��������������Һ��Ũ��Ϊ0.48/233 mol��0.01L=0.21mol/L��0.2mol/L��
a������ʱ��������ƿ��������Һ���ƫС��������һ����Ũ��ƫ��a���ϣ�
b������Ͳȡ2.4mol/LH2SO4��Һʱ���Ӷ�������ȡ������Һ�����ƫС��������Һ��Ũ��ƫС����b�����ϣ�
c���������ˮ���ݣ�ʹ������ƿǰδ�����������ҺŨ����Ӱ�죬��c�����ϣ�
d��ʹ�õ��ձ��Ͳ�����δϴ�ӳ��ף���������ƿ��������������ʵ���ƫС��������ҺŨ��ƫС����d�����ϣ�
e������ʱ������ˮ��������ƿ���棬���Լ�����ˮ����������ҺŨ����Ӱ�죬��e�����ϣ�
�ʴ�Ϊ�����ڣ�A.
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע���c= n/V������Һ��������������

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ
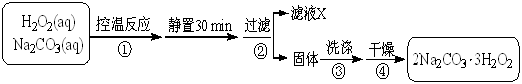
 2Na2CO3��3H2O2 (s) ��H < 0
2Na2CO3��3H2O2 (s) ��H < 0
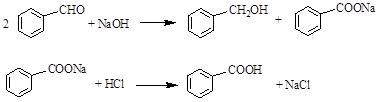



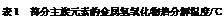
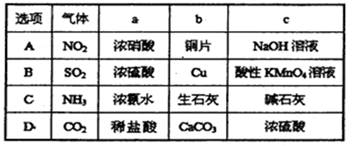
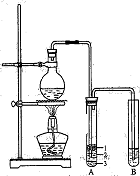
 ��
�� ��H��===2Mn2����5
��H��===2Mn2����5 ��3H2O����ջش����⣺
��3H2O����ջش����⣺