��Ŀ����
����Ŀ����ʮ��������ѧ�����߶�̼����������⻯�����˹㷺������о���ȡ����һЩ��Ҫ�ɹ�����֪��C(s)��O2(g)��CO2(g)����H����393 kJ��mol��1
2CO (g)��O2(g)��2CO2(g)����H����566 kJ��mol��1
2H2(g)��O2(g)��2H2O(g)����H����484 kJ��mol��1
��1����ҵ�ϳ����ý�ˮ�����絽���ȵ�̿����ʵ��ú���������Ƶ�CO��H2�����÷�Ӧ���Ȼ�ѧ����ʽ��_______________��
��2������ú��������������̿�㽻�����������ˮ���������������Ŀ����__________�������������ڼ��Ⱥʹ����ºϳ�Һ��ȼ�ϼ״����÷�Ӧ����ʽΪ_______________��
��3�����ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����⡢�ֽ�������Ӧ�õ���
��һ����2CH3OH(g) === HCOOCH3(g)+2H2(g) ��H>0
�ڶ�����HCOOCH3(g) === CH3OH(g) +CO(g) ��H>0
���Լ״���һ����̼�ķ�ӦΪ___________��Ӧ��������������������������
��4����ѧ���õ����ز�����ͭ��װ��ͼ���˹����ϵͳ�����ø�װ�óɹ���ʵ������
CO2��H2O�ϳ�CH4��
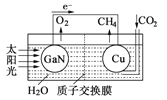
��д��ͭ�缫����ĵ缫��Ӧʽ____________��
��Ϊ��߸��˹����ϵͳ�Ĺ���Ч�ʣ�����װ���м�������___________��ѡ����������������������������
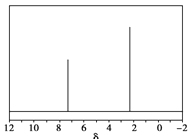
��5����Ȼ��Ҳ��������������ԭ�ϣ������ѧ����������Ȼ��������������÷�����X������������X����Է�������Ϊ106����˴Ź���������ͼ����X�Ľṹ��ʽΪ_____________��
���𰸡�C(s)��H2O(g)= CO (g)��H2(g)����H��+132 kJ��mol��1 �ò���̿ȼ�գ��ṩ̿��ˮ������Ӧ����Ҫ������ CO + 2H2![]() CH3OH ���� CO2��8e����8H����CH4��2H2O ����
CH3OH ���� CO2��8e����8H����CH4��2H2O ���� ![]()
��������
��1����֪����C(s)��O2(g)��CO2(g)����H����393 kJ��mol��1����2CO (g)��O2(g)��2CO2(g)����H����566 kJ��mol��1����2H2(g)��O2(g)��2H2O(g)����H����484 kJ��mol��1�����ݸ�˹���ɣ���-1/2����-1/2���۵�ˮ������̿��Ӧ����CO��H2���Ȼ�ѧ����ʽ��C(s)��H2O(g)="=CO" (g)��H2(g)����H��+132 kJ��mol��1��
��2��ú����Ϊ���ȷ�Ӧ���ʷ�Ӧ����������̿�㽻�����������ˮ���������������Ŀ�����ò���̿ȼ�գ��ṩ̿��ˮ������Ӧ����Ҫ��������CO��H2�ڼ��Ⱥʹ���������Һ��ȼ�ϼ״����÷�Ӧ����ʽΪCO + 2H2![]() CH3OH��
CH3OH��
��3����֪����2CH3OH(g) ==HCOOCH3(g)+2H2(g) ��H>0����HCOOCH3(g) ==CH3OH(g) +CO(g) ��H>0�����ݸ�˹���ɣ���+�ڵ�CH3OH(g) ="=" CO(g)+2H2(g)����H>0�����Լ״���һ����̼�ķ�ӦΪ���ȷ�Ӧ��
��4���������װ��֪��װ��Ϊԭ��أ���ط�ӦΪCO2+2H2O==CH4+2O2��������GaN�缫�����·����ͭ�缫����GaNΪԭ��صĸ�����ͭ�缫Ϊԭ��ص����������ӽ���ĤΪ���ӽ���Ĥ����������ҺΪ������Һ����ͭ�缫Ϊԭ��ص�������������ԭ��Ӧ��CO2�õ������ɼ��飬�����Ϊ������Һ��ͨ�������Ӻ�ˮƽ���ɺ�ԭ�ӣ��缫��ӦʽΪCO2��8e����8H����CH4��2H2O���ڵ����Ϊ������Һ��Ϊ��߸��˹����ϵͳ�Ĺ���Ч�ʣ�����װ���м����������ᡣ
��5��������X����Է�������Ϊ106���������෨�ƶ�X�ķ���ʽΪC8H10����˴Ź���������2���壬X�����к���2����ԭ�ӣ���X�Ľṹ��ʽΪ![]()

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�





