��Ŀ����
����Ŀ��ˮ�帻Ӫ����������ˮ����N��P��Ӫ���κ�������������ˮ����Ⱦ����ȥ��ˮ����N��P�ķ����ܶࡣ
(1)��ѧ������ȥ��ˮ���е�PO![]() �����õĻ�������AlCl3��Ca(OH)2�ȡ�
�����õĻ�������AlCl3��Ca(OH)2�ȡ�
��AlCl3��Һ��Al3+ˮ������ӷ���ʽΪ___��
��Ca5(PO4)3OH��KspΪ6.8��10-37�����ܶȻ���������ʽΪKsp[Ca5(PO4)3OH]=___��
(2)Ϊ̽������ˮ�а�̬��(NH3��N)��PO![]() ͬʱȥ������n(NH4Cl):n(Na2HPO4):n(MgCl2)=1:1:1����ˮ�У�ʵ���ò�ͬpH�£���̬����PO
ͬʱȥ������n(NH4Cl):n(Na2HPO4):n(MgCl2)=1:1:1����ˮ�У�ʵ���ò�ͬpH�£���̬����PO![]() ��ȥ������ͼ(a)��ʾ��
��ȥ������ͼ(a)��ʾ��

��֪��(I)ȥ������Ҫ��ӦΪ��Mg2++NH![]() +HPO
+HPO![]() +6H2O
+6H2O![]() MgNH4PO4��6H2O��+H+��
MgNH4PO4��6H2O��+H+��
(II)����ʱ��Ksp(MgNH4PO4��6H2O)=2.5��10-13��Ksp[Mg(OH)2]=1.8��10-11��Ksp[Mg3(PO4)2]= 6.3��10-31��
��pH��8.5��10.5ʱ����̬����PO![]() ȥ������pH�����������ԭ����___ (��ƽ���ƶ��Ƕ�˵��)��
ȥ������pH�����������ԭ����___ (��ƽ���ƶ��Ƕ�˵��)��
�ڵ�pH��10.5ʱ����̬����PO![]() ȥ������pH�������С����ԭ����___(��ƽ���ƶ��Ƕ�˵��)��
ȥ������pH�������С����ԭ����___(��ƽ���ƶ��Ƕ�˵��)��
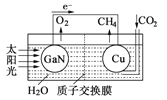
(3)һ�ֽ�ûʽSMDDC��ؿ�����ȥ������ˮ����̬������װ����ͼ(b)��ʾ��

��װ�ù���ʱ��H+��___��(�X����Y")��Ǩ�ơ�
��X����(CH2O)n��CO2�ĵ缫��ӦʽΪ___��Y����NO![]() ��N2�ĵ缫��ӦʽΪ___��
��N2�ĵ缫��ӦʽΪ___��
���𰸡�Al3++3H2O![]() Al(OH)3+3H+ c5(Ca2+)��c3(PO
Al(OH)3+3H+ c5(Ca2+)��c3(PO![]() )��c(OH��) ������H+��OH���������H2O��ʹƽ������ ����Mg(OH)2��Mg3(PO4)2������c(Mg2+)��С��ʹƽ������ Y (CH2O)n+nH2O��4ne-=nCO2��+4nH+ 2NO
)��c(OH��) ������H+��OH���������H2O��ʹƽ������ ����Mg(OH)2��Mg3(PO4)2������c(Mg2+)��С��ʹƽ������ Y (CH2O)n+nH2O��4ne-=nCO2��+4nH+ 2NO![]() +12H++10e-=N2��+6H2O
+12H++10e-=N2��+6H2O
��������
(1)��������ˮ������ӷ���ʽΪAl3++3H2O![]() Al(OH)3+3H+��
Al(OH)3+3H+��
��Ca5(PO4)3OH��ˮ��Һ�д��ڳ����ܽ�ƽ�⣺Ca5(PO4)3OH![]() 5Ca2++3PO
5Ca2++3PO![]() +OH������Ksp[Ca5(PO4)3OH]= c5(Ca2+)��c3(PO
+OH������Ksp[Ca5(PO4)3OH]= c5(Ca2+)��c3(PO![]() )��c(OH��)��
)��c(OH��)��
(2)��ȥ������Ҫ��ӦΪ��Mg2++NH![]() +HPO
+HPO![]() +6H2O
+6H2O![]() MgNH4PO4��6H2O��+H+��pH��8.5��10.5ʱ��������H+��OH���������H2O��ʹƽ�����ƣ���̬����PO
MgNH4PO4��6H2O��+H+��pH��8.5��10.5ʱ��������H+��OH���������H2O��ʹƽ�����ƣ���̬����PO![]() ȥ������pH���������
ȥ������pH���������
��pH����ʱ������Mg(OH)2������ͬʱHPO![]() ����������Ӧ����PO
����������Ӧ����PO![]() ���Ӷ�����Mg3(PO4)2������c(Mg2+)��С��ʹƽ�����ƣ���̬����PO
���Ӷ�����Mg3(PO4)2������c(Mg2+)��С��ʹƽ�����ƣ���̬����PO![]() ȥ������pH�������С��
ȥ������pH�������С��
(3)�پ�ͼ��֪�ŵ������NO![]() ת��ΪN2����ԭ������Y��Ϊ��ص���������X��Ϊ��صĸ�����װ�ù���ʱΪԭ��أ�ԭ������������������ƶ�������H+��Y���ƶ���
ת��ΪN2����ԭ������Y��Ϊ��ص���������X��Ϊ��صĸ�����װ�ù���ʱΪԭ��أ�ԭ������������������ƶ�������H+��Y���ƶ���
��X����(CH2O)nʧ���ӱ���������CO2��H+�����ݵ����غ��Ԫ���غ�ɵõ缫��ӦʽΪ(CH2O)n+nH2O��4ne-=nCO2��+4nH+��Y������NO![]() �õ��ӱ���ԭΪN2�����ݵ����غ��Ԫ���غ�ɵõ缫��ӦʽΪ2NO
�õ��ӱ���ԭΪN2�����ݵ����غ��Ԫ���غ�ɵõ缫��ӦʽΪ2NO![]() +12H++10e-=N2��+6H2O��
+12H++10e-=N2��+6H2O��

����Ŀ��ijѧϰС��̽��Mg��NaHCO3��Һ��Ӧ�Ļ�������������̽����
ʵ��һ��
ʵ��A | ʵ��B | |
���� | ��ע�����м������þƬ(��ȥ��������Ĥ)������15mL����NaHCO3��Һ�� | ��ע�����м������þƬ(��ȥ��������Ĥ)������15mL����ˮ�� |
���� | �����������20min��ע�����ڱں͵ײ�����������ɫ���� | ������������ |
��1��ʵ��B��Ŀ����___��
ʵ�����
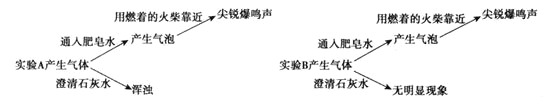
��2������ʵ�����ʵ��A�в�����������___��(�����ʽ)
ʵ�������ֱ�ȡ����ʵ��A��B���ϲ���Һ����֧�Թ��У�������2��BaCl2��Һ��A������������ɫ������B������������
��3��ʵ����˵��Mg��NaHCO3��Һ��Ӧ������___(�����ӷ���)��
ʵ���ģ���С��ͬѧ��ʵ��A�в����İ�ɫ�����������²²⣺����ͼ��ʾװ�ý���ʵ�飬��һ��ȷ����ɫ�����ijɷ֡�

�²�1����ɫ���������ΪMg(OH)2
�²�2����ɫ���������ΪMgCO3
�²�3����ɫ���������Ϊ��ʽ̼��þ[yMg(OH)2xMgCO3]
��4������װ��A��B��Ͻ���ʵ�飬B���а�ɫ���ǣ������___����ȷ����װ�ð�A��C��B��˳����Ͻ���ʵ�飬������___����ʱ������3����ȷ�ġ�
ʵ���壺��ͼ��ʾװ�òⶨ��ʽ̼��þ[yMg(OH)2xMgCO3]����ɣ�
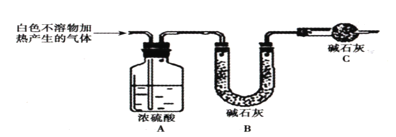
��5����ȡ��������İ�ɫ������7.36g����ּ��������ٲ�������Ϊֹ����ʹ�ֽ����������ȫ������װ��A��B�С�ʵ���װ��A����0.72g��װ��B����2.64g��װ��C��������___����ɫ������Ļ�ѧʽΪ___��