题目内容
将0.2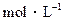 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1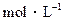 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
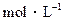 的CH3COONa溶液与0.1
的CH3COONa溶液与0.1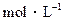 的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是
的HCl溶液等体积混合后,溶液中下列微粒的物质的量浓度的关系中,正确的是| A.[CH3COO-] = [Cl-] = [H+] > [CH3COOH] |
| B.[CH3COO-] = [[Cl-] > [CH3COO-] > [H+] |
| C.[CH3COO-] > [Cl-] > [H+] > [ CH3COOH] |
| D.[CH3COO-] > [Cl-] > [ CH3COOH] > [H+] |
D
略

练习册系列答案
 暑假作业暑假快乐练西安出版社系列答案
暑假作业暑假快乐练西安出版社系列答案
相关题目

 NaCl溶液,可以观察到白色沉淀产生
NaCl溶液,可以观察到白色沉淀产生